SciBase Journals
SciBase Cardiology
ISSN 2691-7785
- Article Type: Case Report
- Volume 1, Issue 1
- Received: Aug 17, 2023
- Accepted: Sep 25, 2023
- Published Online: Oct 02, 2023
Myocardial Involvement in CREST Syndrome: A Case Report of a Rare Myocarditis’ Etiology
Simão Carvalho*; Ana Sofia Azevedo; Adriana Pacheco; Diana Carvalho; Carlos Costa; Tiago Aguiar; Joana Ribeiro; Ana Catarina Faustino; Raquel Ferreira; Andreia Fernandes; Anabela Barcelos; Ana Neves; José Mesquita Bastos
Cardiology Department, Centro Hospitalar Baixo Vouga, Aveiro, Portugal.
*Corresponding Author: Simão Carvalho
Cardiology Department, Centro Hospitalar Baixo Vouga,
Aveiro, Portugal.
Email: almeidacarvalho.simao@gmail.com
Abstract
Limited cutaneous systemic sclerosis also known as CREST syndrome - represents a limited form of systemic sclerosis, and although cardiac involvement is common in systemic sclerosis, it is extremely rare in the limited form [1]. The reported case describes a patient who developed an autoimmune myocarditis, with progression to cardiogenic shock, requiring a multidisciplinary approach in both cardiac and rheumatological diagnosis and treatment. This complex case emphasizes the significance of a multimodal diagnostic approach in identifying the etiology of myocarditis, which has direct implications for therapeutic management.
Keywords: Limited systemic sclerosis; Systemic sclerosis; CREST syndrome; Autoimmune myocarditis; Cardiac magnetic resonance; Endomyocardial biopsy.
Citation: Carvalho S, Azevedo AS, Pacheco A, Carvalho D, Costa C, et al. Myocardial Involvement in CREST Syndrome: A Case Report of a Rare Myocarditis’ Etiology. SciBase Cardiol. 2023; 1(1): 1001.
Introduction
CREST syndrome, recognized as the limited cutaneous variant of systemic sclerosis, represents a complex connective tissue disorder affecting multiple organ systems. The acronym “CREST” encompasses the five primary manifestations, namely: Calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia [2].
In this case report we present a patient who had previously been diagnosed with CREST syndrome and experienced a rapid and significant deterioration of heart function, leading to severe heart failure in a period of two months. The focus of this report is to outline the diagnostic process employed and discuss the therapeutic approach implemented for this challenging scenario.
Case presentation
The patient is a 69-year-old caucasian female with a medical history notable for limited systemic sclerosis/CREST syndrome, secondary biliary cirrhosis and permanent atrial fibrillation. She had been under regular rheumatology consultation, during which her disease manifestations remained stable. These included labial and malar telangiectasis, sclerodactyly, solid alimentary dysphagia and calcinosis affecting the distal interphalangeal joints of three fingers on both hands.
Capillaroscopy revealed characteristic alterations such as dilation and avascular areas. Interestingly, she did not exhibit Raynaud’s phenomenon, which is often associated with CREST syndrome.
Two months earlier she exhibited increasing fatigue with daily life activities and shortness of breath, subsequently evaluated by echocardiography with compatible findings of Heart Failure with Reduced Ejection Fraction (HFrEF), attributed to a non-ischemic etiology. Coronary artery disease was ruled out through coronary angiography. Holter monitor revealed complex ventricular dysrhythmias, specifically non-sustained monomorphic ventricular tachycardias. Cardiac Magnetic Resonance (CMR) hadn’t yet been performed.
The patient was currently receiving treatment, including direct oral anticoagulant, heart failure disease-modifying therapy and prednisolone at a dose of 5 mg once daily.
She presented to the emergency department with a history of progressive exacerbated asthenia and exertional dyspnea on minimal efforts (NYHA functional class III). Upon admission her physical examination revealed a blood pressure of 77/48 mmHg, a heart rate of 82/min, a temperature of 36.8°C, a respiratory rate of 24 cycles per minute and a peripheral oxygen saturation of 97% in room air. Blood tests showed normal hemoglobin and renal function, slightly elevated hepatic cytolysis parameters (AST 123 U/L, ALT 98 U/L), slightly elevated C-reactive protein (1.42 mg/dL) and high-sensitivity troponin I (120.6 pg/mL); brain natriuretic peptide level was markedly elevated (NT-proBNP 17452 pg/mL).
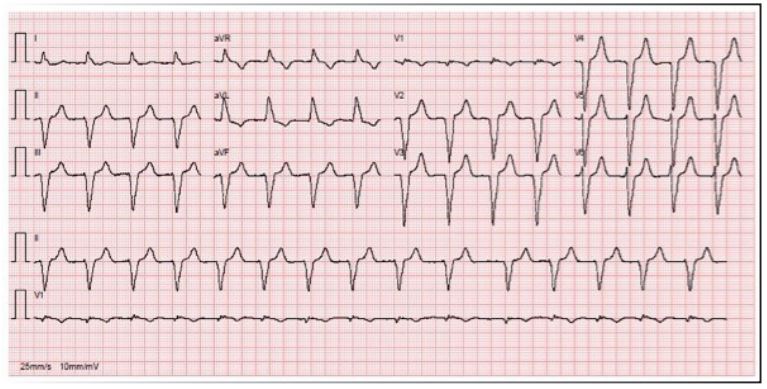
During diagnostic workup an ECG in atrial fibrillation (Figure 1) was performed. Transthoracic echocardiogram revealed severe left ventricular dilation, normal myocardial wall thickness, severe biventricular systolic dysfunction with Left Ventricular Ejection Fraction (LVEF) of 28% and mild to moderate right ventricular dysfunction, with fractional area change of 26%, without segmental contractility abnormalities. Moderate mitral and tricuspid regurgitation and a small pericardial effusion were also observed.
She was admitted to the Intensive Cardiac Care Unit due to decompensated Heart Failure (HF).
The patient’s clinical trajectory included the development of cardiogenic shock, necessitating a comprehensive therapeutic approach involving diuretics, antiarrhythmics, inotropes, vasopressors, and ventilatory support. Moreover, she experienced episodes of type 2 respiratory failure upon minimal exertion and liquid dysphagia, prompting temporary parenteral nutrition.
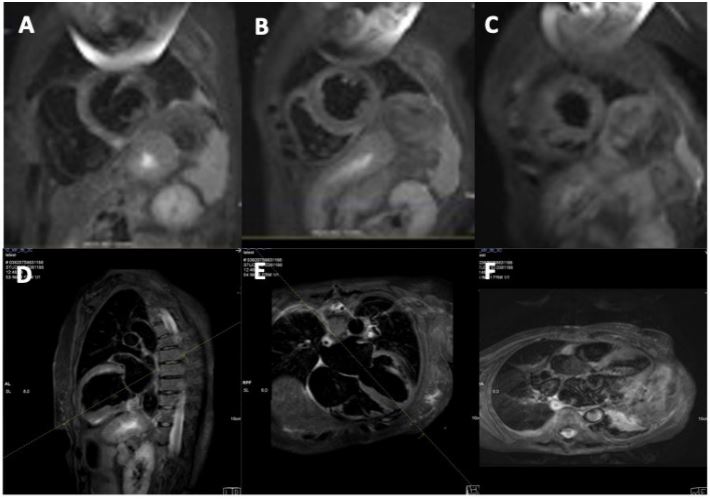
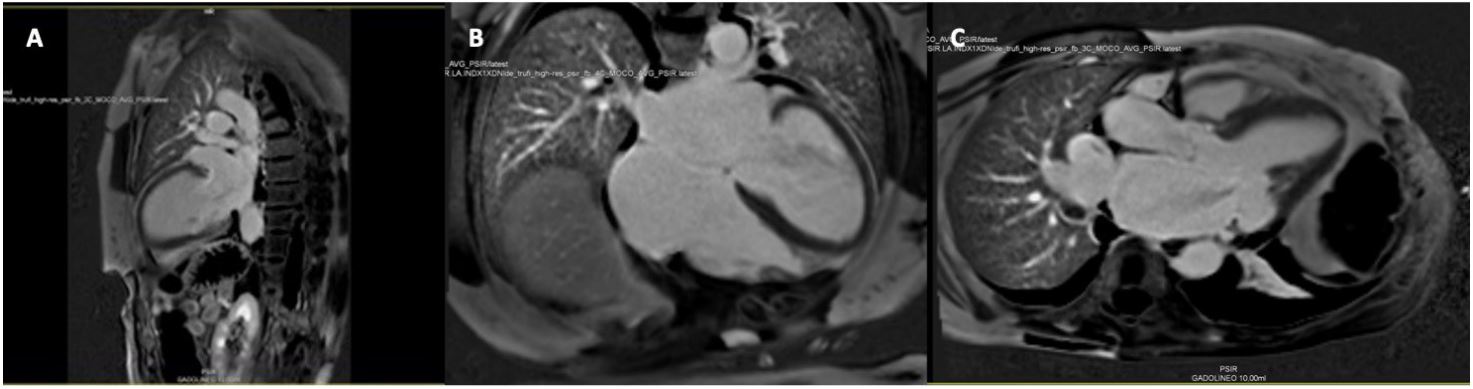
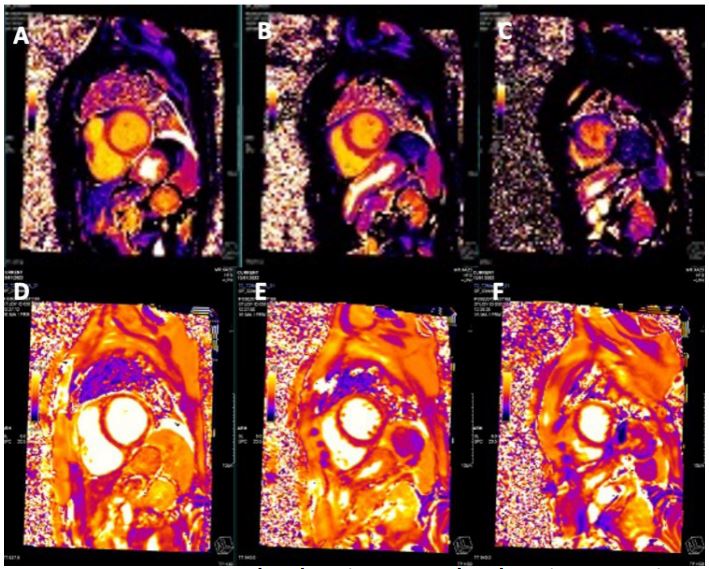
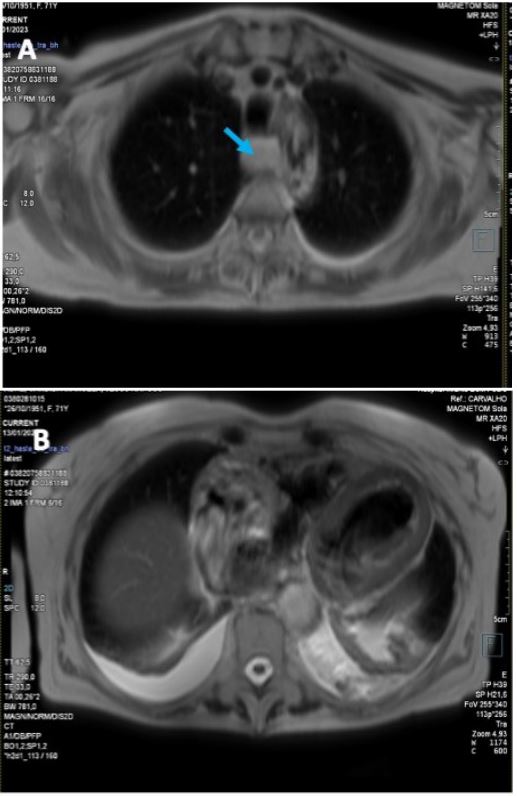
CMR Imaging findings were consistent with echocardiographic observation (Figures 2,3 and 4), showing biventricular dilation and impaired systolic function. Myocardial tissue characterization (T2-weighted sequences) revealed myocardial edema in the basal anteroseptal, anterior, anterolateral, inferoseptal, and inferior segments, with increased T2 mapping values in the basal and mid-ventricular anterolateral, basal inferolateral, mid-anteroseptal, and apical septal segments. Subepicardial late gadolinium enhancement was observed in the basal and mid-segments of the inferior wall, indicating non-ischemic inflammatory cardiomyopathy. Additionally, there was subendocardial late gadolinium enhancement in the basal inferolateral segment and mesocardial striations in the basal and mid-anteroseptal and inferoseptal segments.
Esophageal dilation with liquid content was also noted, consistent with CREST syndrome, and suspicion of inflammatory myositis was raised due to increased T2 signal intensity in the intercostal and pectoral muscles (double the signal intensity of the spinal muscles on T2-weighted sequences).
Given the unfavorable clinical course, an endomyocardial biopsy was performed on the interventricular septum of the right ventricle; five specimens were collected, revealing cardiomyocytes with hypertrophy and lipofuscin pigment, interstitial fibrosis, and mild, focal lymphocytic infiltrate. Viral studies were negative for Coxsackie viruses, adenovirus, influenza virus A and B, parvovirus B19, echovirus, adenovirus, human herpesvirus types 6-8, herpes simplex virus types 1 and 2, human cytomegalovirus, varicella-zoster virus and Epstein-Barr virus confirming the presence of chronic inflammatory changes associated with the rheumatologic disease.
A multidisciplinary meeting was held with Rheumatology and Pathology to discuss the case, leading to the decision to intensify immunosuppressive therapy with increased dose of prednisolone (20 mg) once daily and Mycophenolate Mofetil (MMF) (1000 mg) twice daily was started. The patient showed favorable clinical progression. She was discharged after 28 days of hospitalization, receiving immunosuppressive therapy (prednisolone 15 mg and MMF 1000 mg twice daily) and being referred to a functional rehabilitation program.
Discussion
In Systemic Sclerosis (SSc) cardiac involvement has the potential to impact virtually every component of the heart and is commonly linked with diffuse forms of SSc. The estimated prevalence is 32% and is associated with poor prognosis, contributing to 20-30% of premature mortality cases [3].
In this group of patients, the high frequency of arrhythmias and heart failure has been historically related to histological patchy myocardial fibrosis, associated with concomitant inflammation [4,5].
On the other hand, cardiac involvement in CREST syndrome does occur but is exceedingly rare and less severe [6-9].
The case report highlights the importance of recognizing atypical cardiac presentations in patients with autoimmune disorders.
The diagnostic process presented in the case demonstrates the importance of employing a multimodal approach to unravel the underlying pathology. The patient's symptoms, laboratory results, imaging findings, and histopathological assessmentwere all integrated to establish a comprehensive understanding of the disease process. The use of CMR was particularly instrumental in visualizing the extent of myocardial inflammation and fibrosis, aiding in differentiating non-ischemic inflammatory cardiomyopathy from other potential causes of heart failure [10].
The performance of an endomyocardial biopsy was a critical step in confirming the autoimmune myocarditis and guiding the therapeutic strategy. Traditionally, “New-onset heart failure of 2 weeks to 3 months duration associated with a dilated left ventricle and new ventricular arrhythmias, second or third-degree heart block, or failure to respond to usual care within 1 to 2 weeks” is a criterion to perform EMB [11]. The histopathological examination not only confirmed the presence of myocardial inflammation but also ruled out viral etiologies.
The treatment strategy incorporated not only heart failure prognostic modifying therapy but also immunosuppressive therapy to target the underlying autoimmune component.
In patients with SSc immunosuppressive therapy has the potential risk of scleroderma renal crisis; we equated and minimized that risk through regular renal assessment. Mycophenolate mofetil in combination with steroids has shown a positive effect with normalization of cardiac biomarkers [12] and improved CMR findings in cases of myocarditis in systemic sclerosis [13].
This case exemplifies the value of a multidisciplinary approach in managing complex cases that involve overlapping medical specialties.
Limitations and future research
While this case report provides valuable insights into the management of myocardial involvement in CREST syndrome, it also highlights the rarity and complexity of such cases. Further research and case studies are needed to better understand the underlying mechanisms of autoimmune myocarditis in the context of CREST syndrome and to optimize diagnostic and therapeutic approaches.
Conclusion
In conclusion, this case report presents a compelling example of myocardial involvement in CREST syndrome, highlighting the challenges of diagnosis and management in a patient with overlapping autoimmune and cardiovascular conditions. The successful outcome of this case was a result of meticulous diagnostic workup, collaborative care, and a comprehensive therapeutic strategy.
Conflicts of interest: None declared.
References
- M. Y. M. Y. a. M. H. B. Lesley-Anne Bissell. “Primary myocardial disease in scleroderma-a comprehensive review of the literature to inform the UK Systemic Sclerosis Study Group cardiac working group”, Rheumatology. 2017; 56: 882-895.
- T. K. E. G. M.J. Fritzler, “The CREST syndrome: A distinct serologic entity with anticentromere antibodies,” The American Journal of Medicine. 1980; 69: 520-526.
- B. B. V. M. A. P. C. F. C. P. e. a. Tyndall AJ. “auses and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database”, Ann Rheum Dis. 2010; 69: 1809-15.
- R. R. S. W. H. G. Bulkley BH. “Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction.,” Circulation. 1976; 53: 483-90.
- M. I. E. D. Z. C. S. P. K. U. K. I. R. M. K. R. G. M. G. T. H. J. K. K. Mueller KA. “Clinical and histopathological features of patients with systemic sclerosis undergoing endomyocardial biopsy”, PLoS One. 2015; 10: 5.
- W. P. F. Anita Deswal, “Cardiac Involvement In Scleroderma,” Rheumatic Disease Clinics of North America. 1996; 22: 841-860.
- W. F. Akesson A. “Organ manifestations in 100 patients with progressive systemic sclerosis: A comparison between the CREST syndrome and diffuse scleroderma,” Br J Rheumatol. 1989; 28: 281.
- A. K. a. Y. Allanore, “Primary myocardial involvement in systemic sclerosis,” Rheumatology. 2006; 45: iv14-iv17.
- G. K. M. A. J. P. K. P. A. P. D. S. W. J. Handa R. “Cardiac involvement in limited systemic sclerosis: Non-invasive assessment in asymptomatic patients,” Clin Rheumatol. 1999; 18: 136-9.
- J. S. M. &. M. C. Lagan. “Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases,” Int J Cardiovasc Imaging. 2018; 34: 35-54.
- K. L. B. A. M. F. A. F. M. J. U. K. G. N. L. J. N. R. C. S. J. T. R. V. Leslie T. Cooper. “The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease,” Circulation. 2007; 116: 2216- 2233.
- DSMZ G, e a Pieroni M. “Recognizing and treating myocarditis in recent-onset systemic sclerosis heart disease: Potential utility of immunosuppressive therapy in cardiac damage progression”. 2014; 43: 2014.