SciBase Journals
SciBase Cardiology
ISSN 2996-4547
- Article Type: Case Series
- Volume 2, Issue 1
- Received: Jan 10, 2024
- Accepted: Feb 20, 2024
- Published Online: Feb 27, 2024
Advanced Cardiac Conduction Abnormalities in Patients on Ibrutinib Therapy
Romil Patel*; Charlie Robin; Jason Robin
Northshore University Health system, Graduate School of Biomedical Sciences, Rowan University School of Osteopathic Medicine, Stratford, NJ 08084 USA.
*Corresponding Author: Romil Patel
Email: romil.patel12@outlook.com
Abstract
Background: Bruton tyrosine kinase inhibitors, such as ibrutinib, are medications that are actively used for Chronic Lymphocytic Leukemia (CLL) and several lymphomas. They work by inhibiting Bruton’s tyrosine kinase therefore affecting B cell proliferation. Ibrutinib itself is associated with multiple cardiotoxicities including atrial fibrillation, ventricular arrhythmias, heart failure, and bleeding. There are well-established studies that show the incidence of atrial fibrillation with ibrutinib therapy. However, only a few reports show an association between ibrutinib therapy and advanced heart block.
Case presentation: We present two unique cases of patients who presented to the emergency room with syncope while being on ibrutinib therapy for CLL. At presentation, the patients were found to have conduction abnormalities seen on their EKG that included complete heart block in one patient and sick sinus syndrome in the other. Ultimately, each patient was treated with a pacemaker, ibrutinib therapy was discontinued, and they were started on alternative therapy for their CLL.
Discussion: No clear mechanism has been established for how ibrutinib therapy causes these conduction abnormalities. This case series proposes a possible mechanism of advanced conduction abnormalities that is caused by ibrutinib therapy that is associated with the NOTCH gene. We propose that the downregulation of the NOTCH gene may prevent cardiac myocyte remodeling and therefore can cause conduction abnormalities as seen in our patients along with others that presented with advanced heart block with ibrutinib therapy.
Keywords: Bruton tyrosine kinase inhibitors; Ibrutinib, Advanced heart block; NOTCH Gene.
Abbreviations: CLL: Chronic Lymphocytic Leukemia; EKG: Electrocardiogram; BCR: B-Cell Receptor
Citation: Patel R, Robin C, Robin J. Advanced Cardiac Conduction Abnormalities in Patients on Ibrutinib Therapy. SciBase Cardiol. 2024; 2(1): 1007.
Introduction
Bruton tyrosine kinase inhibitors, such as ibrutinib, are medications that have been replacing chemotherapy-based regimens in patients with Chronic Lymphocytic Leukemia (CLL) and some lymphomas [1]. These medications achieve therapy by inhibiting Bruton’s tyrosine kinase which is a mediator of the B-Cell Receptor (BCR) signaling pathway that affects B-cell proliferation and differentiation [2]. However, when these medications are used for the long term, therapy can be complicated by adverse cardiovascular side effects [1].
Ibrutinib itself is associated with multiple cardiotoxicities including atrial fibrillation, ventricular arrhythmias, heart failure, and bleeding. Previous studies have shown that the incidence of atrial fibrillation has been seen anywhere between 5-16% of patients depending on their duration of therapy. Patients who develop atrial fibrillation on ibrutinib therapy are managed medically with rate control medications and initiation of anticoagulation, while keeping in mind that ibrutinib therapy has an increased bleeding risk, however therapy can continue for these patients. Other studies demonstrate ventricular arrhythmias arising with patients on ibrutinib therapy and in some cases, leading to cardiac death [3-8].
In this report, we specifically focus on ibrutinib therapy causing cardiac conduction disorders, specifically high-degree heart block and sinus arrest. Per the study by Vartanov et al. there have been at least 18 cases with reported high-degree heart blocks [3]. Here we present two unique cases from our institution.
Case series
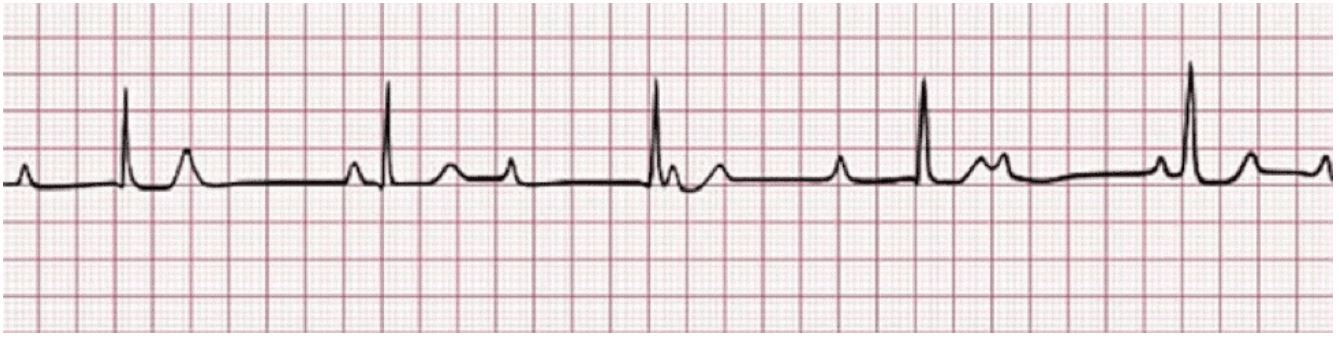
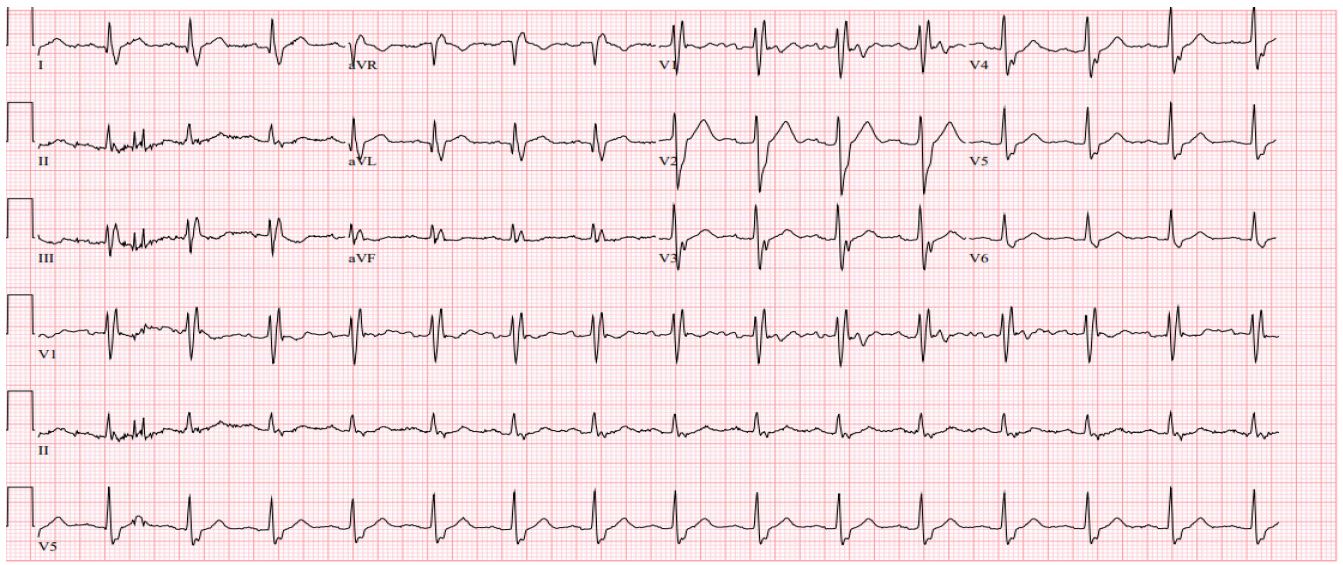
Patient 1 is a 68-year-old male with a history of small lymphocytic lymphoma that was confirmed with a lymph node biopsy. The patient was first diagnosed in 2007 and was under observation until 2009 when systemic symptoms presented. He was initially trialed on Rituximab and alemtuzumab but had disease progression. He had multiple other treatments through the years with some improvement in disease course but ultimately had further progression. Finally, in 2014, the patient was started on ibrutinib therapy which he tolerated well except for some fatigue. In January 2020, the patient developed atrial fibrillation while on ibrutinib and was started on apixaban. Later that year in November, he suddenly became unresponsive while watching a football game at home. The patient woke up on his own but did not recall the syncopal episode. When EMS arrived, the patient was found to be in a 3rd-degree AV block with a heart rate in the low 30s on their cardiac monitor strip done by EMS (Figure 1). When getting to the hospital, a repeat EKG was done in the emergency room after the patient had self-converted to a normal sinus rhythm with a right bundle branch block (Figure 2). However due to the syncopal episode and reported thirddegree heart block by EMS, the patient was admitted to the hospital and ultimately treated with a pacemaker on the day of admission. The patient remained stable during admission without further episodes of heart block. He ultimately stopped ibrutinib therapy and was started on alternative therapy due to persistent episodes of atrial fibrillation and rapid ventricular response.
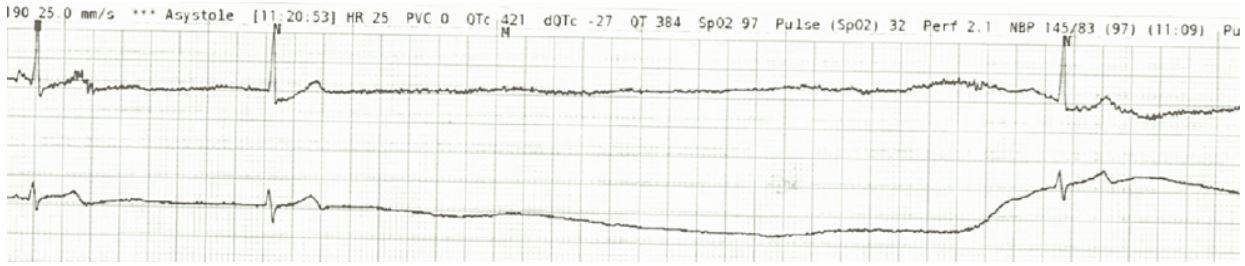
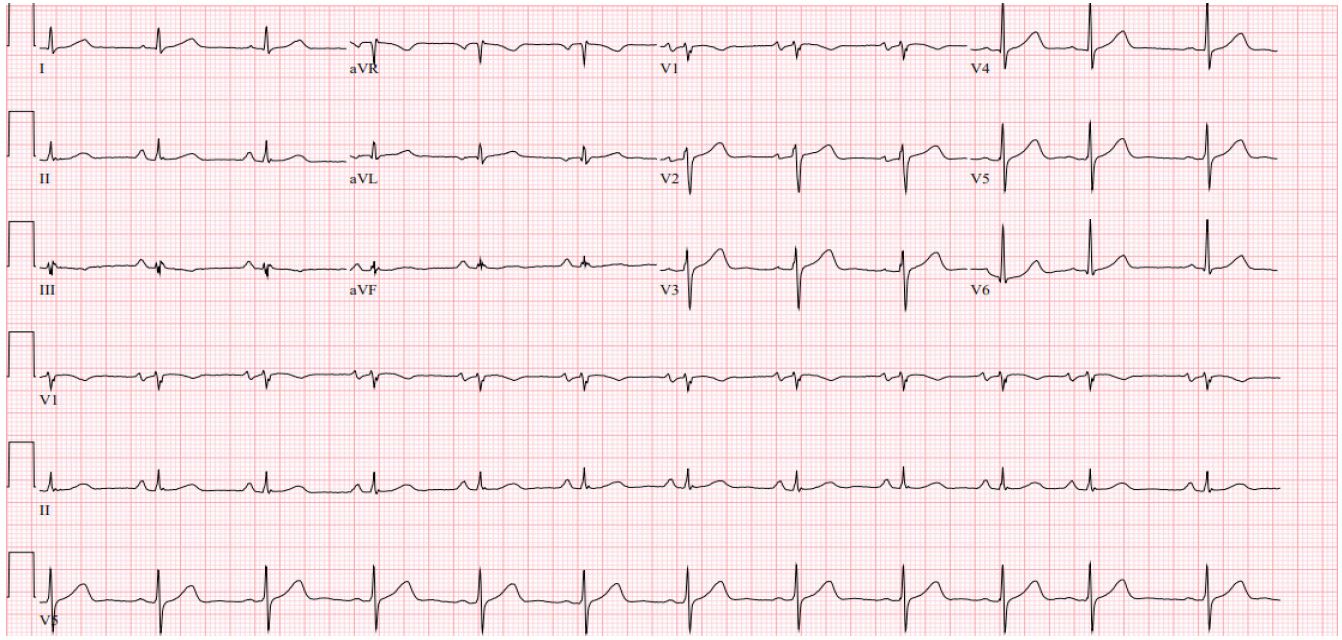
Patient 2 is a 71-year-old female who was diagnosed with CLL in 2015. She was started on ibrutinib/Rituxan treatment at that time. In May of 2021, the patient had a syncopal episode at home and when presenting to the emergency room, was found to be in atrial fibrillation with rapid ventricular response seen on EKG. The patient was admitted for further observation and management and had a normal echocardiogram and stress test. However, during this hospitalization, she had prolonged episodes of sinus pause seen on telemetry that led to further syncopal episodes during the admission (Figure 3). The longest pause that was seen on telemetry was 40 seconds. It was determined that the patient had sick sinus syndrome in addition to atrial fibrillation. Therefore, before discharge, the patient had a permanent pacemaker placed. Following pacemaker placement, the patient had no further syncopal or sinus pause events but did have further episodes of atrial fibrillation with rapid response and was treated with dofetilide and apixaban. Ultimately, the patient converted to normal sinus rhythm and was discharged on dofetilide and apixaban for anticoagulation (Figure 4). Therapy was changed to acalabrutinib on follow-up with oncology following discharge.
Discussion
This case series shows two patients with no prior significant cardiac history who presented to the hospital with advanced conduction abnormalities after being on ibrutinib therapy. Based on previous studies, patients on ibrutinib therapy have a well-known risk of developing atrial fibrillation. Incidence of atrial fibrillation can be as high as 5-16% as shown earlier in this article [3-8]. However, newer case series and studies are showing a possible relation between ibrutinib therapy and conduction disease. Typically, in these patients, therapy would be paused until a pacemaker is placed but can be continued thereafter or changed to an alternative therapy as this is what happened to our patients. At this time, there is no clear mechanism that has been found to relate ibrutinib therapy with advanced conduction abnormalities.
One mechanism that we propose for the advanced conduction abnormalities related to ibrutinib therapy is based on the NOTCH gene regulation. Bruton’s tyrosine kinase inhibitors cause BCR blockade which causes decreased activation of the NOTCH gene [10]. Studies have shown that the NOTCH gene is involved in the modulation of cardiomyocyte survival, cardiac stem cell differentiation, and angiogenesis [11]. Activation of NOTCH signaling has been shown to improve cardiac function, minimize myocardial fibrosis, suppress cardiomyocyte apoptosis, and increase neovascularization to improve outcomes after myocardial infarction [12]. As a result, the downregulation in the NOTCH gene due to BCR blockade can modulate atrioventricular canal myocardial remodeling as well as ventricular remodeling which is associated with deterioration of cardiac performance and potentially increased fibrosis [10,11,13]. It has also been seen that alterations in the NOTCH signaling can modulate atrioventricular canal remodeling which can lead to conduction defects in cardiac cells [9,10]. We propose that the downregulation of the NOTCH gene can lead to increased cardiac cell apoptosis and myocardial fibrosis that in turn leads to poor cardiac remodeling that ultimately causes conduction abnormalities due to continued use of ibrutinib therapy. This mechanism is what we propose caused the advanced conduction abnormalities in our patients who were on ibrutinib therapy which led to their hospital presentations after syncopal episodes.
Ultimately the treatment for these patients would be the insertion of a pacemaker. Our patients after pacemaker placement had no further syncopal episodes. Therapy with ibrutinib could be continued after pacemaker insertion, however, in both of our patients, therapy was changed to an alternative medication.
Conclusion
Our study here shows two patients at our facility who presented with advanced conduction abnormalities requiring permanent pacemaker placement. These cases along with the previous others described in the study by Vartanov et al show a new adverse effect to monitoring while patients are on ibrutinib therapy [3]. No clear mechanism has been established for the development of these advanced conduction abnormalities. Here we demonstrate the importance of the NOTCH signaling within cardiac cells and propose a possible relation of the downregulation of the NOTCH gene due to ibrutinib therapy causing advanced conduction abnormalities. Future studies will be needed to isolate the NOTCH gene to find a direct relation between the inhibition of the NOTCH gene and conduction abnormalities.
Our study is limited in the number of patients as we only have two unique cases of advanced conduction abnormalities due to ibrutinib therapy at our institution. It is also limited as we cannot test the relation between NOTCH inactivation and conduction abnormalities in an isolated setting by single gene knockout. Future studies hopefully have the opportunity to look at this relation to determine if there is a cause and effect relation between these.
References
- Burger, Jan A. Bruton Tyrosine Kinase Inhibitors. The Cancer Journal. 2019; 25(6): 386-393, https://doi.org/10.1097/ppo.0000000000000412.
- D Rozkiewicz, et al. Bruton’s Tyrosine Kinase Inhibitors (BTKIs): Review of Preclinical Studies and Evaluation of Clinical Trials. Molecules. 2023; 28(5): 2400–2400, https://doi.org/10.3390/molecules28052400.
- Vartanov, Alexander R, et al. High-Grade Heart Block Associated with Ibrutinib Therapy. HeartRhythm Case Reports, 2021; 7(6): 391-394. www.ncbi.nlm.nih.gov/pmc/articles/PMC8226304/, https://doi.org/10.1016/j.hrcr.2021.03.013. Accessed 17 Dec. 2023.
- Dong, Rong, et al. Ibrutinib-Associated Cardiotoxicity: From the Pharmaceutical to the Clinical. Drug Design Development and Therapy. 2022; 16: 3225-3239. www.ncbi.nlm.nih.gov/pmc/articles/PMC9508996/, https://doi.org/10.2147/dddt.s377697. Accessed 3 Dec. 2023.
- Husam Abdel-Qadir, et al. Cardiovascular Risk Associated with Ibrutinib Use in Chronic Lymphocytic Leukemia: A PopulationBased Cohort Study. Journal of Clinical Oncology. 2021; 39(31): 3453-3462. https://doi.org/10.1200/jco.21.00693. Accessed 9 Aug. 2023.
- Salem, Joe-Elie, et al. Cardiovascular Toxicities Associated with Ibrutinib. Journal of the American College of Cardiology. 2019; 74(13): 1667-1678. www.sciencedirect.com/science/article/pii/S0735109719361558,https://doi.org/10.1016/j.jacc.2019.07.056. Accessed 14 Oct. 2019.
- Lampson, Benjamin L, et al. Ventricular Arrhythmias and Sudden Death in Patients Taking Ibrutinib. Blood. 2017; 129(18): 2581-2584. https://doi.org/10.1182/blood-2016-10-742437. Accessed 6 Feb. 2023.
- Brown Jennifer R, et al. Characterization of Atrial Fibrillation Adverse Events Reported in Ibrutinib Randomized Controlled Registration Trials. Haematologica. 2017; 102(10): 1796-1805. haematologica.org/article/view/8228, https://doi.org/10.3324/haematol.2017.171041. Accessed 13 Jan. 2023.
- de la Pompa, José Luis, and Jonathan A. Epstein. Coordinating Tissue Interactions: Notch Signaling in Cardiac Development and Disease. Developmental Cell. 2012; 22(10): 244-254. https://doi.org/10.1016/j.devcel.2012.01.014. Accessed 19 May 2020.
- Beatrice Del Papa, et al. Decreased NOTCH1 Activation Correlates with Response to Ibrutinib in Chronic Lymphocytic Leukemia. Clinical Cancer Research, 2019; 25(24): 7540-7553. https://doi.org/10.1158/1078-0432.ccr-19-1009. Accessed 11 Nov. 2023.
- Ferrari, Roberto, and Paola Rizzo. The Notch Pathway: A Novel Target for Myocardial Remodelling Therapy? European Heart Journal. 2014; 32(32): 2140-2145. academic.oup.com/eurheartj/article/35/32/2140/2481269, https://doi.org/10.1093/eurheartj/ehu244. Accessed 17 Dec. 2023.
- Li Yuxin, et al. Notch Signaling as an Important Mediator of Cardiac Repair and Regeneration after Myocardial Infarction. Trends in Cardiovascular Medicine, 2010; 20(7): 228-231, https://doi.org/10.1016/j.tcm.2011.11.006. Accessed 11 May 2019.
- Niessen, Kyle, and Aly Karsan. Notch Signaling in Cardiac Development. Circulation Research. 2008; 102(10): 1169-1181. https://doi.org/10.1161/circresaha.108.174318. Accessed 12 Nov. 2019.