SciBase Journals
SciBase Cardiology
ISSN 2996-4547
- Article Type: Research Article
- Volume 2, Issue 1
- Received: Feb 10, 2024
- Accepted: Mar 15, 2024
- Published Online: Mar 22, 2024
Effect of Combined Oral Contraceptive Pills on Established Biomarkers of Lipid Metabolism: Systematic Review and Meta-Analysis
Amazon Doble*; Nicola King
University of Plymouth, UK.
*Corresponding Author: Amazon Doble
Email: Amazon.doble@plymouth.ac.uk
Abstract
Background and aims: Bruton tyrosine kinase inhibitors, such as ibrutinib, are medications that are actively used for Chronic Lymphocytic Leukemia (CLL) and several lymphomas. They work by inhibiting Bruton’s tyrosine kinase therefore affecting B cell proliferation. Ibrutinib itself is associated with multiple cardiotoxicities including atrial fibrillation, ventricular arrhythmias, heart failure, and bleeding. There are well-established studies that show the incidence of atrial fibrillation with ibrutinib therapy. However, only a few reports show an association between ibrutinib therapy and advanced heart block.
Methods: A systematic review was carried out involving searching in research databases including PubMed using key search terms. This was followed by a meta-analysis that compared cholesterol, high density lipoprotein, low-density lipoprotein and triglycerides in a population taking combined oral contraceptive pills containing ethinyl oestradiol/levonorgestrel at 6 months vs the baseline measurement.
Results: Six studies (9 intervention groups), totalling 274 participants were analysed. Hedge’s g for the cholesterol comparison was 0.133 (95% confidence intervals -0.058, 0.324; p=0.173; I2 =74.652), which was not significant. In contrast high density lipoprotein was significantly lower, Hedge’s g -0.546 (95% confidence intervals -0.834, -0.259; p<0.001; I2 =92.546) and lowdensity lipoprotein significantly higher, Hedge’s g 0.248 (95% confidence intervals 0.055, 0.442; p=0.012; I2 =83.116 as were the triglycerides, Hedge’s g 0.667 (95% confidence intervals 0.491, 0.842; p<0.001; I2 =64.627.
Conclusion: After 6 months use the combined oral contraceptive pills containing ethinyl oestradiol/levonorgestrel led to a significantly increased concentration of low-density lipoprotein and triglycerides and a significantly lower concentration of high-density lipoprotein. More research is required to investigate whether these changes are associated with an increased risk of cardiovascular disease.
Keywords: Atherosclerosis; Ethinyl estradiol; levonorgestrel; cholesterol; HDL; LDL.
Citation: Doble A, King N. Effect of Combined Oral Contraceptive Pills on Established Biomarkers of Lipid Metabolism: Systematic Review and Meta-Analysis. SciBase Cardiol. 2024; 2(1): 1008.
Introduction
Cardiovascular diseases are a group of disorders of the heart and blood vessels, including Aoronary Artery Disease (CAD), which is responsible for 16% of the world’s total deaths [1]. Atherosclerosis is the forerunner to CAD and in severe cases when blood flow through the coronary arteries is obstructed a myocardial infarction can occur. Factors such as: diet, smoking, hyperglycaemia, hypercholesterolemia and hyperlipoproteinemia can influence the risk of developing atherosclerosis [2].
Oral Contraceptives (OCs) are the most popular method of female short-acting birth control and in 2019 were used by 151 million females (aged 15-49) worldwide [3]. OCs simple administration and low side effect incidence have instigated widespread use and confidence in this method of pregnancy prevention. OCs comprise synthetic forms of reproductive hormones, which suppress ovulation and implantation that would normally facilitate pregnancy. Endogenous reproductive hormones in women include oestrogen and progesterone, however, the synthetic forms used within OCs include ethinyl-estradiol and progestins (levonorgestrel, norethindrone and desogestrel [4]. The first line choice for women starting on the Combined Oral Contraceptive Pill (COCP) normally contain ethinyl-estradiol and levonorgestrel [5].
Sex hormones within the body have been shown to possess influential effects on the production of lipoproteins, cholesterol and total triglyceride levels. Endogenous and synthetic oestrogens increase High-Density Lipoprotein (HDL) levels, while nortestosterone-derived progestins have been shown to lower HDL levels and progesterone derivatives have little effect on HDL [4]. Oestrogen has been shown to have protective effects against atherosclerosis by reducing levels of Low-Density Lipoprotein (LDL). However, there is comparatively less known concerning the cardiovascular actions of progesterone and progestins, yet dose-dependent progesterone has been observed to inhibit the beneficial effect of oestrogen within experimental atherosclerosis, suggesting progesterone can exert an inhibitory action on oestrogen-protective mechanisms, potentially affecting the hormones associated with atherosclerosis and COCP [7].
Glisic, et al. [8] conducted a meta-analysis that investigated progestin-only OCs with cardiometabolic outcomes comprising of heart attack and stroke, concluding no associated risk of cardiometabolic outcomes. In contrast, this meta-analysis aims to evaluate whether COCP use affects a female’s lipoprotein metabolism. This involves a comparison of the baseline value vs the value at 6 months in healthy women taking a COCP containing Ethinyl-Estradiol (EE) and Levonorgestrel (LNG). It is hypothesised that the women will have a changed level of biomarkers at the 6 month time point indicating a greater risk of developing atherosclerosis.
Methods
Ethical approval: This study is a systematic review and metaanalysis. This involves the pooling of data from already published work only. As such it does not require ethical approval or the approval of an institutional board. It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Search approach: Potential studies were identified using systematic searches, carried out using the PubMed, CENTRAL and Web of Science databases. The search strategy included the key terms of “oral contraception” AND “atherosclerosis” AND “Lipids”. Searches of published papers were conducted up until the end of September 2021. All identified papers were assessed independently by the two authors.
Included and excluded trials: Only Randomized Controlled trials (RCTs) and prospective trials of participants taking a combined ethyl-oestradiol levonorgestrel contraceptive pill were included. Other hormone-related methods of birth control, such as the implant, patch, shot, ring and intrauterine device, were excluded from the trial. Other combinations of COCP were excluded. Only studies containing outcomes of lipoprotein metabolism products were included. Animal studies, review papers and retrospective trials were excluded from this study and there were no language boundaries. Studies that did not have any of the desired outcome measures were excluded. Studies that did not contain any control data (pre-treatment cycles or control cycles) were excluded. Also, incomplete data, or data from an already included study (duplicate study) was excluded. Studies involving women with obesity, polycystic ovarian syndrome and diabetes were excluded.
Participants/population: RCTs and prospective trials of healthy female adult participants aged 18-50 were included. The populations included were from studies ranging from 6 months to 13 months duration.
Intervention(s), exposure(s): This meta-analysis studied all RCTs and prospective trials where woman were taking a COCP containing ethinyl-estradiol and levonorgestrel.
Control/Comparator (s): This meta-analysis used RCT’s and prospective trials that compared COCP with ethinyl-estradiol and levonorgestrel pre-treatment/control cycles to an end point value at 6 months on lipid metabolism biomarkers.
Search results: Our initial search found 3,652 articles. Of these 3,569 studies were excluded on the basis of title and abstract. 63 studies were excluded as they were not RCTs or prospective trials. Of the RCTs and prospective trials we excluded 15 studies: 2 studies that contained duplicate data; 5 studies involving women with polycystic ovarian syndrome; 5 studies containing the wrong pill combination; 1 study that focussed on the inflammatory profile; 1 study involving women with hypercholesterolaemia; 1 study involving women with obesity and 1 study that focussed on insulin sensitivity (see supplementary Figure S1 and Table S1). Six studies (9 intervention groups) were included in our analysis [9,14].
Outcome(s): The primary outcomes analysed were cholesterol level; HDL-C level; LDL-C level and triglyceride level.
Risk of bias (quality) assessment: A modified JADAD scale was used in assessing study quality and reporting throughout the meta-analysis [15]. Other bias including publication bias were investigated using funnel plots.
Strategy for data synthesis: Meta-analyses were completed for continuous data by calculating Hedge’s g between the baseline measurement and the 6month time point for each study recorded. A random effects model was used. Heterogeneity was quantified using I2 [16], 95% confidence intervals were used; significance was assumed at p<0.05; and, figures were produced using Complete Meta-analysis (CMA version 3). Where a single trial had 2 interventions using 2 different concentrations of ethinyl oestradiol and levonorgestrel, these are referred to as low and high in the forest plots. Where values were reported as medians and ranges these were converted to mean and standard deviation using the calculations derived by Hozo et al. [17].
Results
The 6 studies (9 intervention groups) included in the pooled analyses had an aggregate of 274 participants. Table 1 summarises the characteristics of the included studies. Supplementary Table S1 lists the excluded RCTs and reasons for exclusion.
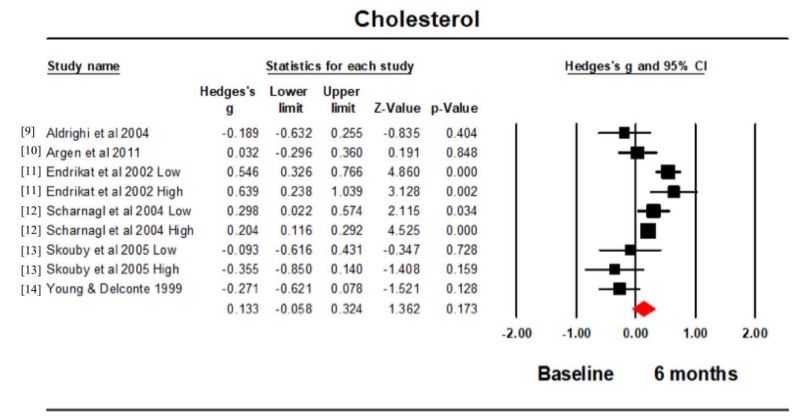
Cholesterol: All 6 studies (9 intervention groups) reported the cholesterol concentration at baseline and 6 months. Hedge’s g for the comparison was 0.133 (95% confidence intervals (CI) -0.058, 0.324; p=0.173; I2 =74.652) (Figure 1). There was no significant difference between the cholesterol concentration at baseline and that at 6 months. The funnel plot was symmetrical (Figure 2).
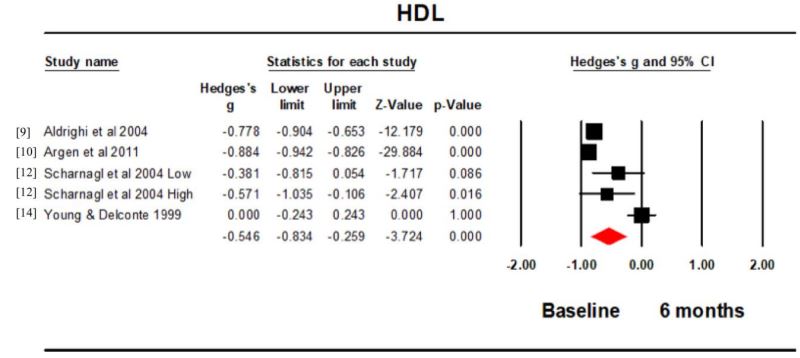
HDL: Four studies (5 intervention groups) measured the HDL concentration at baseline and 6 months. Hedge’s g for the comparison was -0.546 (95% CI -0.834, -0.259; p<0.001; I2 =92.546) (Figure 2). The HDL concentration was significantly higher at baseline compared to 6 months. The funnel plot was funnel shaped but not symmetrical (Figure 3).
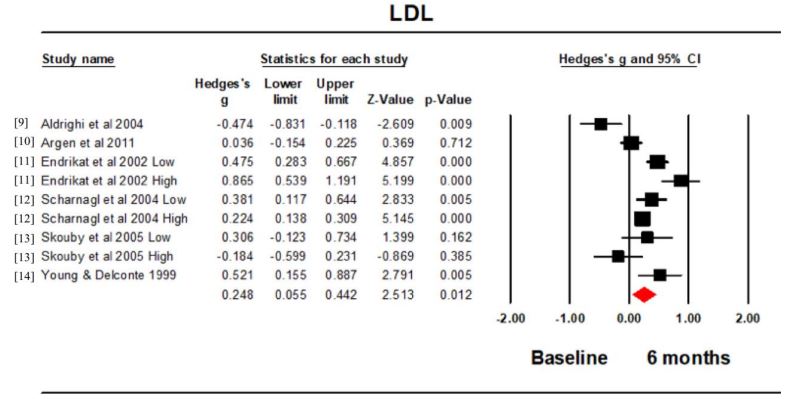
LDL: All 6 studies (9 intervention groups) reported the LDL concentration at baseline and 6 months. Hedge’s g for the comparison was 0.248 (95% CI 0.055, 0.452; p=0.012; I2 =83.116) (Figure 3). The LDL concentration at 6 months was significantly larger than that at baseline. The funnel plot was symmetrical (Figure 4).
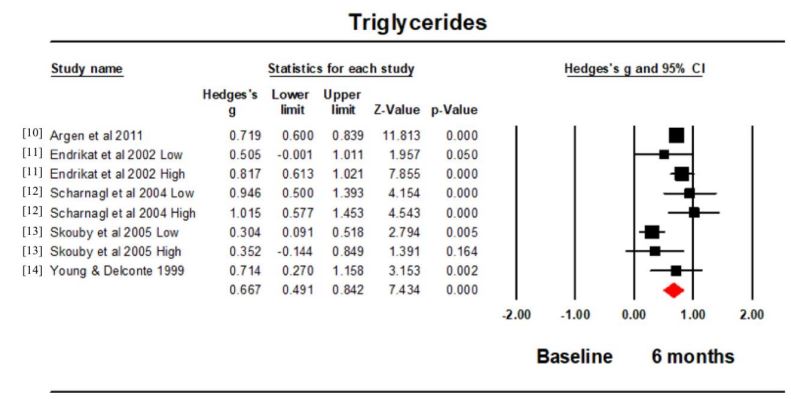
Triglycerides: Five studies (8 intervention groups) reported the triglyceride concentration at baseline and 6 months. Hedge’s g for the comparison was 0.667 (95% CI 0.491, 0.842; p<0.001; I2 =64.627) (Figure 4). The triglyceride concentration was significantly higher at 6 months compared to the baseline. The funnel plot was symmetrical.
Footnotes: CI: Confidence Interval.
Footnotes: HDL: High Density Lipoprotein; CI: Confidence Interval.
Footnotes: LDL: Low Density Lipoprotein; CI: Confidence Interval.
Footnotes: CI: Confidence Interval.
Table 1: Characteristics of included studies.
| Study ID | EE/LNG concentration | Length of study | n | Age (years)* | Outcomes |
|---|---|---|---|---|---|
| Aldrighi et al. 2004 [9] |
30 μg/50 μg for 6 days 40 μg/75 μg for 5 days 30 μg/125 μg for 10 days |
6 months | 29 | 37.3±2 |
Cholesterol HDL LDL |
| Argen et al. 2011 [10] | 30 μg/150 μg for 21 days | 6 months | 52 | 29.1±7.8 |
Cholesterol HDL LDL Triglycerides |
|
Endrikat et al. 2002 [11]
low Endrikat et al. 2002 [11] high |
20 μg/100 μg for 21 days 30 μg/150 μg for 21 days |
13 months 13 months |
23 25 |
22.7(18-27)** 24.2(18-32)** |
Cholesterol LDL Triglycerides |
|
Scharnagl et al. 2004 [12]
low Scharnagl et al. 2004 [12] high |
20 μg/100 μg for 21 days 30 μg/150 μg for 21 days |
12 months 12 months |
34 33 |
27±4 26±5 |
Cholesterol HDL LDL Triglycerides |
|
Skouby et al. 2005 [13]
low Skouby et al. 2005 [13] high |
20 μg/100 μg for 21 days 30 μg/150 μg for 21 days |
13 months 13 months |
22 27 |
23.5(range 19-28) 24.1(range 20-30) |
Cholesterol LDL Triglycerides |
| Young & Delconte 1999 [14] | 20 μg/100 μg for 21 days | 12 months | 28 | 32.9±5.8 |
Cholesterol HDL LDL Triglycerides |
Footnotes: *Mean and standard deviation; **Median and range; EE: Ethinyl Estradiol; LNG: Levonorgestrel; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein.
Discussion
A raised plasma concentration of cholesterol, LDL and triglycerides is a well-known risk factor for cardiovascular disease in women [18]. It is therefore concerning that reports exist linking changes in lipid metabolism to the use of the Combined Oral Contraceptive Pill (COCP) [19,20]. These reports though refer to the COCP in general rather than a specific formulation. The results showed when comparing baseline (before treatment) with 6 months after treatment began the EE/LNG combination led to a significantly increased concentration of LDL and triglycerides, a significantly lower concentration of HDL and no change in the cholesterol concentration. These results suggest that changes to lipid metabolism can occur with the EE/LNG combination and that perhaps as a result caution should be advised for women with other cardiovascular risk factors.
The overall effect size showed there to be no significant difference in the cholesterol concentration over the measured time period. This was also the case for the effect size for the individual studies except Endrikat et al. [11] and Scharnagl et al . [12], which both showed a significantly higher effect size for the comparison between the 2 time points. This occurred regardless of the EE/LNG concentration suggesting this was independent of the EE concentration. It is not possible to discern if there were differences in the assay conditions in the different trials, because sometimes these were not mentioned [11]. The majority of studies were carried out in Europe with one in Brazil and one in the USA. There was also a higher plasma concentration of cholesterol in COCP users compared to non-users in studies carried out in Iran and Finland, although these either do not precisely state which COCP [19] were being used, or grouped different kinds of COCP into one [20].
The overall effect size for HDL suggested a reduction in the concentration from baseline to 6 months. This was also true for each of the individual studies, except Young and Delconte [14]. This study is the only one carried out pre 2000 and it is possible there may have been advances in assay technique between this and the later studies.
There was a significant increase in the LDL concentration at 6 months compared to baseline. The one very clear exception to this was the study by Aldrighi et al. [9], where the LDL concentration decreased at 6 months. This difference could have been due to the different dosing regime used in this study compared to the others. All the other studies used a monophasic dosing regime, whereas Aldrighi et al. [9] used a triphasic dosing regime. This triphasic dosing regime varied the concentration of EE and LNG from 30-40 µg and 50-125 µg respectively, which may have then exerted a differential effect on the LDL concentration. Unfortunately there were insufficient trials to conduct a subgroup analysis on the effect of different dosing regimes. This could however become a focus for further investigations.
All of the studies, which investigated it, showed an increase in triglycerides at 6 months. This was reflected in a significant effect size. Although this may fit in with a generalised picture of dyslipidaemia associated with possible increased cardiovascular risk, triglyceride concentrations are not the best biomarker in determining atherosclerosis association [21]. Elevated triglycerides levels are more commonly correlated with acute pancreatitis [22]. An interesting association to investigate as oestrogenic dose-dependent increases can be reflected by a stimulation of Very-Low-Density Lipoprotein (VLDL) assembly, primarily due to an enlarged hepatic production of triglycerides. Though oestrogen administration has been demonstrated in previous research to raise serum triglycerides, further research would be beneficial in explicitly investigating this oral contraceptive relationship [23]. Moreover, this significant result may be attributed to total triglyceride levels including counts for other fractions of lipoproteins such as apolipoproteins AI, AII and B, also fractions of LDL such as VLDL [24]. These fractions were not measured within this meta-analysis due to limited literature available, however, they also possess atherogenic properties, as displayed in animal models treated with OC’s and fed an atherogenic diet. These models showed increases in LDL and VLDL cholesterol, large reductions in HDL-C and increased plasma cholesterol, indicating a predisposition towards atherosclerosis [25].
Limitations: Hormonal contraception can be delivered by several routes including oral, injection, interuterine and patch. This meta-analysis is limited to just considering oral administration. The formulation of the COCP also offers many different choices as to hormone combination and dosing regime. This meta-analysis has solely focussed on the EE/LNG combination, which is the first line choice in the UK [5].
The gold standard studies to include in a meta-analysis are Randomised Controlled Trials (RCTs). This involves a comparison of two groups, which are matched at baseline. This was the case for 4 of the included studies in this meta-analysis [10,13]. Unfortunately, 2 of the studies were not RCTs and only before and after values for a single group were available [9,14]. This contributed to a low score according to the modified Jadad scale and may have introduced unwanted bias. Otherwise, the median modified Jadad score was moderate.
Clinical relevance: The most recent other meta-analysis regarding oral contraceptive pill use on lipid metabolism from Silva-Bermudez, et al. released in 2020 [26]. However, this study focused on all oral contraceptive pills, not specifically combined oral contraceptive pills. They also included studies looking at PCOS and endometriosis potentially altering the metabolism pathway occurring in healthy individuals [26]. Also, given that the combination of Ethinyl Estradiol (EE) and Levonorgestrel (LNG) is the normal first line choice [5], it was considered important for our study to investigate the effects of this combination on plasma lipid levels in a meta-analysis of randomised controlled clinical trials and prospective trials. The novelty of this meta-analysis stems from our inclusion of only combined oral contraception pills, allowing us to look at the metabolism of the most frequent risk groups at first to provide information to the largest population group. The clinical significance of our analysis provides further support for the proposed relationship of contraceptive pills, specifically combined oral contraceptive pills to the risk of acute pancreatitis, given our identification of significantly increase triglyceride levels across these included studies [22].
Conclusion
After 6 months use the oral combined contraceptive pill containing EE/LNG led to a significantly increased concentration of LDL and triglycerides and a significantly lower concentration of HDL in the blood. More research is required to investigate whether these changes are associated with an increased risk of cardiovascular disease.
Acknowledgments: None.
Funding: This work did not receive any funding.
References
- WHO (World Health Organisation), (2021). Cardiovascular diseases, Health topics. World Health Organisation. 2021. Available from: https://www.who.int/health-topics/cardiovasculardiseases#tab=tab_1 (Accessed: 01/10/21).
- Singh R, Mengi S, Xu Y, Arneja A, Dhalla, N. (2002). Pathogenesis of atherosclerosis: A multifactorial process. Exptl Clin Cardiol. 2002; 7: 40-53.
- UN (United Nations), (2019). Contraceptive use by Method 2019: Data booklet. United Nations, Department of Economic and Social Affairs, Population Division. 2019. Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf(Accessed: 01/10/21).
- Tikkanen M, Nikkilä E. Oral contraceptives and lipoprotein metabolism. J Reproduct Med. 1986; 9: 898-905.
- BNF (2021). Contraceptives, hormonal. Available from: https://bnf.nice.org.uk/treatment-summary/contraceptives-hormonal.html (accessed 01/10/2021).
- Mendelsohn M, Karas R. The Protective Effects of Estrogen on the Cardiovascular System. New Engl J Med. 1999; 340: 1801-11.
- Hanke H, Bruck B, Brehme U, Gugel N, Finking G, Haasis R. Inhibition of the protective effect of estrogen by progesterone in experimental atherosclerosis. Atherosclerosis, 1996; 121: 129-38.
- Glisic M, Shahzad S, Tsoli S, Chadni M, Asllanaj E, Rojas LZ et al. Association between progestin-only contraceptive use and cardiometabolic outcomes: A systematic review and meta-analysis. Eur J Preventive Cardiol. 2018; 25: 1042-52.
- Aldrighi JM, Petta CA, Bahamondes L, Caetano ME, Martinez TRL, Rodrigues G et al. Lipid profile in women over 35 years old taking triphasic combined oral contraceptives. Contraception. 2004; 69: 395-9.
- Gren UM, Anttila M, Mäenpää-Liuko K, Rantala M-L, Rautiainen H, Sommer WF et al. Effects of a monophasic combined oral contraceptive containing levonorgestrel and ethinylestradiol on haemostasis, lipid and carbohydrate metabolism. Eur J Contraception Reproduct Health. 2011; 16: 444-57.
- Endrikat J, Klipping C, Cronin M, Gerlinger C, Ruebig A, Schmidt B et al. An open label, comparative study of the effects of a dosereduced oral contraceptive containing 20µg ethinyl estradiol and 100µg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception. 2002; 65: 215-21.
- Scharnagl H, Petersen G, Nauck M, Teichmann AT, Wieland H, März W. Double-blind, randomized study comparing the effects of two monophasic oral contraceptives containing ethinylestradiol (20µg or 30µg) and levonorgestrel (100µg or 150µg) on lipoprotein metabolism. Contraception. 2004; 69: 105-13.
- Skouby SO, Endrikat J, Düsterberg B, Schmidt W, Gerlinger C, Wessel J et al. A 1-year randomized study to evaluate the effects of a dose reduction in oral contraceptives on lipids and carbohydrate metabolism: 20µg ethinyl estradiol combined with 100µg levonorgestrel. Contraception. 2005; 71: 111-7.
- Young RL, Delconte A. Effects of low-dose monophasic levonorgestrel with ethinyl estradiol preparation on serum lipid levels: A twenty-four-month clinical trial. Am J Obstet Gynecol. 1999; 181: 559-62.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al. Assessing the Quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17: 1-12.
- Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al: The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. Br Med J. 2011; 343: d5928.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method 2005; 5: 13.
- Samson ME, Adams SA, Merchant AT, Maxwell WD, Zhang J, Bennett CL et al. Cardiovascular disease incidence among females in South Carolina by type of oral contraceptives, 2000-2013: a retrospective cohort study. Arch Gynecol Obstet. 2016; 294: 991-7.
- Momeni Z, Dehgani A, Fallahzadeh H, Koohgardi M, Dafei M, Hekmatimoghaddam SH et al. The impacts of pill contraceptive low-dose on plasma levels of nitric oxide, homocysteine, and lipid profiles in the exposed vs. non exposed women: as the risk factor for cardiovascular diseases. Contraception Reproduct Med. 2020; 5: 7.
- Wang H, Garruti G, Liu M, Portincasa P, Wang D. Cholesterol and Lipoprotein Metabolism and Atherosclerosis: Recent Advances in Reverse Cholesterol Transport. Ann Hepatol. 2017;16: 21-36.
- Brown T, Bittner V. Biomarkers of atherosclerosis: clinical applications. Current cardiology reports. 2008; 10: 497-504.
- Kota S, Kota S, Jammula S, Krishna S, Modi K. Hypertriglyceridemia-induced recurrent acute pancreatitis: A case-based review. Ind J Endocrin metab. 2012; 16: 141-3.
- Knehtl M, Bevc S. Oral contraception-induced hypertriglyceridemia pancreatitis: A case report of a rare but still present complication. J Dis Markers. 2014; 1: 1015.
- Lavie C. Lipid and Lipoprotein Fractions and Coronary Artery Disease. Mayo Clin Proc 1993; 68: 618-9.
- Sissan M, Leelamma S. Role of oral contraceptive in atherosclerosis. Ind J Exptl Biol. 1994; 32: 307-10.
- Silva-Bermudez, L., Toloza, F., & Perez-Matos, M. Effects of oral contraceptives on metabolic parameters in adult premenopausal women: a meta-analysis. Endocrine Connections. 2020; 9(10): 978-998.
Table S1: Analysis of study quality (Modified Jadad scale).
| Study |
Random Sequence Generation |
Allocation Concealment |
Blinding |
Selective Reporting |
Intention to Treat Analysis |
Incomplete Outcome Data |
Groups Matched at Baseline |
Total |
|---|---|---|---|---|---|---|---|---|
| Aldrighi et al 2004 [9] | NA | NA | NA | NO | NO | NO | NA | 2 |
| Argen et al 2011 [10] | YES | Unclear | NO | NO | NO | NO | YES | 4 |
| Endrikat et al 2002 [11] | YES | Unclear | NO | NO | NO | NO | YES | 4 |
| Scharnagl et al 2004 [12] | YES | YES | YES | NO | NO | NO | YES | 6 |
| Skouby et al 2005 [13] | YES | Unclear | NO | NO | NO | NO | YES | 4 |
| Young & Delconte 1999 [14] | NA | NA | NA | NO | NO | NO | NA | 2 |
| TOTALS | 4/6 | 1/6 | 1/6 | 6/6 | 0/6 | 6/6 | 4/6 | Median 4 |