SciBase Journals
SciBase Clinical and Medical Case Reports
ISSN 2691-7785
- Article Type: Research Article
- Volume 1, Issue 2
- Received: Sep 05, 2023
- Accepted: Oct 20, 2023
- Published Online: Oct 27, 2023
Characteristics and Management of Brucellosis-Related Spondylodiscitis: A Prospective Study
Makram Koubaa1*; Fatma Hammami1 ; Fatma Smaoui1 ; Khaoula Rekik1, ; Wiem Feki2 ; Nour Ben Ayed3; Chakib Marrakchi1 ; Mounir Ben Jemaa1
1Infectious Diseases Department, Hedi Chaker University Hospital, University of Sfax, Tunisia.
2Department of Radiology, Hedi Chaker University Hospital, University of Sfax, Tunisia.
3Microbiology Laboratory, Habib Bourguiba University Hospital, University of Sfax, Tunisia.
*Corresponding Author: Makram Koubaa
Infectious Diseases Department, Hedi Chaker University Hospital, Sfax 3029, Tunisia.
Tel: +216-21-880 402 & +216-74-264 906;
Email: koubaa_makram@medecinesfax.org
Abstract
Introduction: Brucellosis-Related Spondylodiscitis (BSD) presents diagnostic and therapeutic challenges due to nonspecific clinical signs, delayed appearance of radiological findings, and the lack of precise therapeutic consensus.
Objective: This study aimed to determine the epidemiological, clinical, biological, radiological, therapeutic, and outcome characteristics of BSD.
Patients and methods: A prospective study was conducted, including all patients hospitalized in the infectious diseases department between 2011 and 2020 with a confirmed diagnosis of BSD. Diagnosis was established based on a combination of clinical, biological, and radiological evidence.
Result: The study comprised 41 cases. The mean age of patients was 52.2±15.7 years, with a male predominance (71%). Consumption of contaminated dairy products (90.2%) was the primary mode of transmission. The average time to consultation was 101.5±68 days. Febrile spinal pain was reported in all cases. Wright serology was positive in all cases. Brucella melitensis was isolated in 5 blood cultures (12.8%). The lumbar spine was the most commonly affected site (65.8%). Reported complications included paravertebral abscesses (61%), psoas abscesses (29.3%), epiduritis (61%), and spinal cord compression (14.6%). The most commonly used antibiotic combination was doxycycline-rifampicin-trimethoprim-sulfamethoxazole (39%). The mean treatment duration was 7.3±3.1 months. Favorable outcomes were observed in 63.1% of cases.
Conclusions: Brucellosis-related spondylodiscitis remains a public health concern in endemic countries, warranting the implementation of strategies for eradicating animal brucellosis and establishing safety and hygiene measures for human prevention.
Keywords: Spondylodiscitis; Brucella; Wright’s agglutination; Rifampicin; Tetracyclines; Prophylaxis.
Citation: Koubaa M, Hammami F, Smaoui F, Rekik K, Feki W, et al. Characteristics and Management of Brucellosis-Related Spondylodiscitis: A Prospective Study. SciBase Clin Med Case Rep. 2023; 1(2): 1008.
Introduction
Brucellosis, also known as Malta fever, melitococcosis, sudoralgic fever, and Mediterranean undulant fever [1], is an anthropozoonosis endemic in Mediterranean regions, the Middle East, Asia, Central and South America, and Africa [2,3]. Classic reservoirs of the bacteria are livestock animals, and transmission occurs through direct contact with infected animals or their biological products, as well as through the ingestion of raw milk and derivatives [4,5]. In developed countries, brucellosis is considered an occupational disease with low prevalence due to widespread infection control in animals and milk pasteurization. However, in developing countries, it remains a significant public health concern due to its high prevalence, impact on both animal productivity and human health, leading to work absenteeism, high expenses for diagnosis, treatment, and eradication programs [6,7].
Brucellosis is a systemic infection characterized by a wide clinical polymorphism. Osteoarticular involvement accounts for 10 to 80% of all focal forms of brucellosis [8]. Among these, Brucellosis-Related Spondylodiscitis (BSD) is the most frequent osteoarticular localization, comprising 2 to 65% of cases [9]. BSD poses diagnostic and therapeutic challenges due to its nonspecific symptoms, delayed appearance of radiological anomalies compared to clinical symptoms, and the absence of a welldefined therapeutic consensus.
The objectives of our study were to:
Describe the epidemiological, clinical, biological, and radiological aspects of BSD.
Discuss the circumstances and diagnostic difficulties associated with BSD.
Detail the therapeutic modalities and outcomes of BSD.
Patients and methods
We conducted a prospective study including all patients hospitalized in the infectious diseases department between 2011 and 2020 with a confirmed diagnosis of BSD. The diagnosis of BSD was established based on a combination of clinical, biological, and radiological evidence [7,10,11]:
Clinical data consistent with the diagnosis.
Spinal involvement confirmed through imaging.
Detection of specific antibodies using Wright’s agglutination test (WAT) with a titer greater than 1/160 and/or isolation of Brucella from blood or other specimens.
Results
Epidemiology
During the study period, a total of 157 patients were admitted to the infectious diseases department with a diagnosis of brucellosis, of which osteoarticular brucellosis was identified in 49 patients. BSD was confirmed in 41 patients, representing a frequency of 26.1% of all brucellosis cases and 83.7% of osteoarticular involvements. Within the same timeframe, 142 patients were hospitalized for infectious spondylodiscitis, with a brucella origin identified in 41 patients, resulting in a frequency of 28.9%. The peak of hospitalizations was observed in October (7 cases). Contamination predominantly occurred in the summer for 14 patients (34.1%).
We included 29 males (71%) and 12 females (29%). The male-to-female ratio was 2.42. The mean age of patients was 52.2±15.7 years, with the highest frequency observed in the age group between 50 and 60 years (31.7%). Contact with domestic animals was reported in 35 patients (85.4%). Sixteen patients (39%) were farmers. The consumption of raw milk and/ or unpasteurized dairy products was reported in 37 patients (90.2%). Family history of brucellosis was described by 8 patients (19.5%).
Clinical study
The average time from onset of symptoms to consultation was 101.5±68 days, with a range of 21 to 330 days. The primary reason for consultation was spinal pain associated with fever in all patients. Fever was reported by all patients during the course of their illness. Sweating was described by 31 patients (75.6%), mainly occurring at night and with an unpleasant odor.
The main osteoarticular symptom was spinal pain, which was present in all patients. Other symptoms included arthralgia (43.9%), myalgia (24.4%), and sacroiliac pain (12.2%). A temperature above 38°C was recorded in 22 patients (53.7%). Tenderness at the spinous processes and spinal stiffness were common (83% and 58.5% respectively). Paravertebral muscle spasm was present in 20 patients (48.8%), and pain in the sacroiliac joints was noted in 8 patients (19.5%). Neurological deficits were observed in 16 patients (39%), including motor deficits in 10 patients (24.4%), with 2 of them having cervical involvement. Sensory deficits were noted in 14 patients (34.1%). Two patients (4.8%) experienced urinary problems in the form of dysuria, and 1 patient had abolished osteotendinous reflexes (2.4%). Among patients with motor deficits, epiduritis and spinal cord compression were noted in 6 and 2 cases respectively.
Hepatomegaly and splenomegaly were found upon admission in 2 and 4 patients respectively. Axillary lymphadenopathy was reported in 1 patient (2.4%). Additionally, orchitis and sacroiliitis were associated with 2 patients (4.8%) and 4 patients (9.7%) respectively.
Biological study
Anemia was found in 9 patients (21.9%). Leukopenia was present in 4 patients (9.7%). Erythrocyte Sedimentation Rate (ESR) was measured in 39 patients, with an average of 50.4 ± 31.5 mm at the first hour. C-Reactive Protein (CRP) was measured in 33 patients, and it was positive in 27 patients (81.8%), with an average value of 49.6±61.3 mg/L. Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) levels were measured in 39 patients (95.1%). Elevated ALT and AST levels above 40 IU/L were observed in 8 patients (20.5%) and 9 patients (23.1%) respectively. Gamma-Glutamyl Transferase (GGT) levels were measured in 27 patients (65.8%). Hepatic cholestasis was noted in 3 patients (11.1%).
Blood Cultures (BC) were performed in 39 patients (95.1%), and they were positive in 5 patients (12.8%). The isolated microorganism was Brucella melitensis in all cases. Among the 12 patients who presented with psoas abscess, Brucella testing in the aspirated abscess fluid was conducted in 7 patients, and it was negative in all cases. Wright’s Agglutination Test (WAT) was performed for all patients and was positive in all cases, with titers ranging from 1/160 to 1/10240. Rose Bengal test was conducted in 40 patients (98%), and it was positive in 100% of cases.
Spinal Disc-Vertebral Biopsy (DVBD) was performed in 11 patients (26.8%). Histopathological examination revealed inflammatory changes in 7 patients (63.6%) and paravertebral abscess without specificity or signs of malignancy in 2 patients (18.2%). In our study, Brucella identification was not performed using the PCR technique. Moreover, DVBD showed caseous necrosis in 2 patients (18.2%).
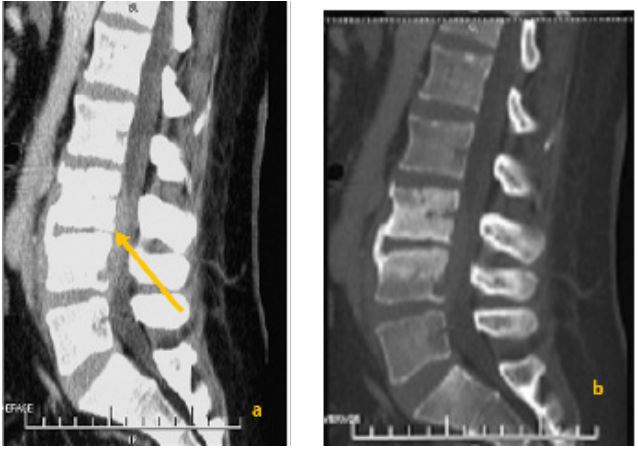
Radiological study
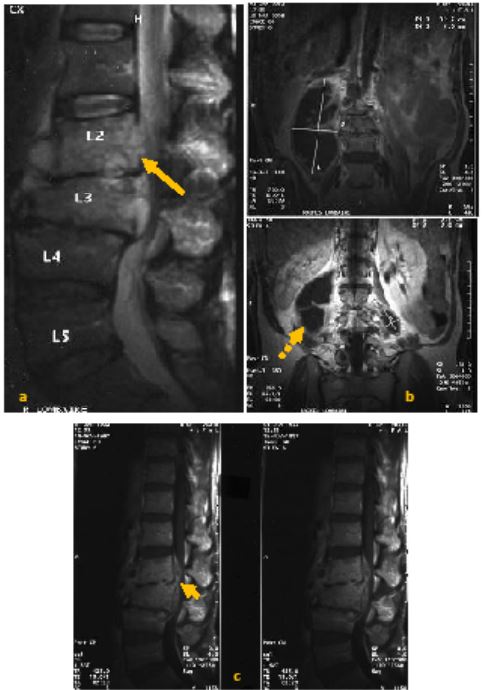
All patients underwent standard spine radiography. Disc space narrowing was observed in 40 patients (97.6%). Erosion of the anterosuperior angle was noted in 10 patients (24.4%). Twenty-six patients underwent spinal Computed Tomography (CT) (63.4%) (Figure 1). CT revealed vertebral endplate erosions in all patients. Hypodensity of the intervertebral disc was observed in 22 patients (84.6%). Magnetic Resonance Imaging (MRI) was performed on 32 patients (78%) (Figure 2). The disc showed hypointensity on T1-weighted images in 31 patients (96.9%) and hyperintensity on T2-weighted images in 30 patients (93.7%). Vertebral endplates were hypointense on T1-weighted and hyperintense on T2-weighted images in 29 patients (90.6%), enhancing after gadolinium injection in 27 patients (84.4%).
Overall, the radiological assessment revealed epiduritis in 25 patients (61%), paravertebral abscess in 25 patients (61%), psoas abscess in 12 patients (29.3%), and spinal cord compression in 6 patients (14.6%).
Bone scintigraphy was performed in 12 patients (29.3%). It showed increased uptake at the affected vertebrae in 10 patients (75%), associated with sacroiliac joint uptake in 3 patients (25%).
Single-level involvement was observed in 30 patients (73.1%), of which 4 had multifocal involvement spanning ≥3 vertebrae (13.3%). The lumbar spine was the most frequently affected site (65.8%). Contiguous involvement was noted in 3 patients: the thoracolumbar junction was affected in 2 patients with concurrent thoracic or lumbar involvement (1 case each). One patient had involvement of the lumbar and lumbosacral regions. Simultaneous involvement of the cervical, thoracic, and lumbar regions was observed in 1 patient (Table 1).
Treatment
Rifampicin along with a tetracycline (doxycycline and oxytetracycline) were prescribed for all patients. Cotrimoxazole was prescribed for 16 patients (39%), and only 4 patients (9.7%) were treated with ciprofloxacin (Table 2). The average duration of treatment was 7.3±3.1 months, ranging from 3 to 16 months. Corticosteroids were prescribed for 1 patient (2.4%) who presented with bilateral radicular compression.
Among the 12 patients with psoas abscess, radioguided abscess drainage was performed in 7 patients (58.3%). Indications for drainage were based on the significant size of the abscess, with an average size of 10.2±2.9 cm, and lack of regression under medical treatment. No patients underwent surgical abscess drainage. Nine patients received anti-tuberculosis treatment in combination with anti-brucellar treatment for an average duration of 12.2±4.7 months, ranging from 7 to 23 months. Antituberculosis treatment was initiated in 5 patients directly, of whom 4 had motor deficits.
The indication for this treatment was based on:
The destructive nature of lesions observed in spinal MRI (1 patient).
Positive tuberculin skin test (Mantoux test) in 3 patients and/or positive detection of Koch’s bacillus in 1 patient.
Caseous necrosis revealed by DVBD in 2 patients or in a biopsy of a dorsal cutaneous fistula in 1 patient.
We introduced a trial of anti-tuberculosis treatment in 3 patients due to the lack of improvement in clinical symptoms with anti-brucellar treatment alone. One patient was treated with both anti-tuberculosis and anti-brucellar treatments due to therapeutic relapse characterized by bilateral cruralgia and right lower limb weakness.
All patients were advised to use pain relievers and antipyretics, as well as to rest.
Outcome
The mean follow-up duration was 36±5 months. Favorable outcomes were observed in 24 patients (63.1%). All patients achieved afebrile status within an average of 3.7±2.9 days, with disappearance of spinal pain and absence of anatomical or functional sequelae. On the biological level, CRP levels normalized within an average of 59.9±57.5 days, ranging from 4 to 180 days, and follow-up imaging showed significant improvement in spinal lesions.
Sequels were observed in 14 patients (36.8%). These consisted of spinal pain in 12 patients (31.6%) and/or spinal stiffness in 1 patient (2.6%). Sensory deficits in the form of paresthesias were present in 3 patients (7.9%). These paresthesias were bilateral in 2 patients and unilateral in 1 patient. No patients retained residual motor deficits. No cases of death were observed in our study.
Table 1: Levels of spinal involvement in brucellar spondylodiscitis.
| Level | Number | Percentage (%) |
|---|---|---|
| Cervical | 4 | 9.7 |
| Thoracic | 7 | 17.1 |
| D12-L1 | 3 | 7.3 |
| Lumbar | 27 | 65.8 |
| L5-S1 | 5 | 12.2 |
Table 2: Different antibiotic combinations used in the management of brucellar spondylodiscitis.
| Number | Percentage (%) | |
|---|---|---|
| Rifampin + Doxycyline + Cotrimoxazole | 16 | 39 |
| Rifampin + Doxycycline | 14 | 34.2 |
| Rifampin + Oxytetracycline | 7 | 17.1 |
| Rifampin + Doxycycline + Ciprofloxacin | 4 | 9.7 |
Discussion
Epidemiology Brucellosis is a globally distributed zoonosis, predominantly prevalent in Mediterranean regions, the Middle East, Asia, Central and South America, and Africa [12,13]. According to the World Health Organization (WHO), around 500,000 cases of human brucellosis are reported each year [14,15]. However, its true incidence is often underestimated and varies from country to country. It has significantly declined in developed countries [12,16]. Nevertheless, the incidence of human brucellosis varies in endemic areas, ranging from 0.1 to 200 cases per 100,000 population per year [15,17]. These variations can be attributed to high endemicity of animal brucellosis, increased animal import rates in developing countries [18], and the absence of an effective brucellosis control strategy [7,19].
Brucellar spondylodiscitis constitutes 3 to 29% of all brucellosis cases [10,13,20,21], and 2 to 68% of brucellosis-related osteoarticular infections [9,22-25]. Moreover, brucellosis accounts for 17 to 48% of all cases of spondylodiscitis in Turkey and Spain [26-28], whereas it represents only 0.4% in France [29,30].
Clinical study
Patients usually consult for spinal pain, which was reported in 92 to 100% of the cases [10,31]. Systemic symptoms were reported among BSD cases, including fever, which has an undulant characteristic [12,32], nocturnal and malodorous sweating, asthenia, weight loss and anorexia [14,33]. In our study, fever was reported among all patients and sweating among 75.6% of the cases. As for the osteoarticular signs, they are generally represented by spinal pain, arthralgia, myalgia [34,35] and sometimes by pain in the sacroiliac joints in the event of associated sacroiliitis.
In fact, during brucellosis there is a succession of different phases. Following the incubation period during which the bacterium multiplies, Brucella colonizes the cells of the reticuloendothelial system, which constitutes the acute phase which is manifested by an undulant suduro-algic fever. Later on, the disease might progress to a subacute phase characterized by the alleviation of fever due to the partial control of the infection by the immune system and by the appearance of secondary sites of the disease. When the evolution is beyond one year, brucellosis evolves towards a chronic phase [36,37]. Therefore, brucellosis is characterized by myriad and non-specific symptoms, whether it’s associated by osteoarticular sites or not.
Biological study
The diagnosis of BSD might be confirmed by isolation of Brucella by culture or by nucleic acid amplification assays which enable rapid diagnosis of the disease. Otherwise, serological tests remain the primary tools for the diagnosis and post-therapeutic follow-up of brucellosis [38]. In fact, the isolation of Brucella can be done from different biological samples such as the intervertebral disc, bone tissue or paravertebral abscess during surgery or by aspiration or by percutaneous discovertebral biopsy [39]. These interventional invasive procedures were required among 0% to 8.5% among series [40]. Therefore, blood cultures and serological tests are of immense help and thus reduce the need for interventional procedures in order to confirm the diagnosis.
Radiological study
As for the radiological evaluation, MRI remain the diagnostic method of choice in spondylodiscitis, epidural abscess and cord or root compression relevant to brucellosis. In MRI, the lesion is found as destructive appearance at antero-superior corner of vertebrae accompanied by prominent osteosclerosis, which is a pathognomonic finding during brucellosis [39].
In default, when MRI is not available, CT scan might help during the diagnostic process. The affected intervertebral disc appears hypodense due to edema and abscess. This is a reliable sign of disc infection [41]. The involvement of the soft tissue results in an obliteration of the fatty plans. An abscess appears as a rounded lesion with a hypodense center and a hyperdense peripheral crown which is strongly enhanced after injection of contrast product [41]. Epidural involvement is also better explored with the injection of contrast products [9].
Treatment
The aims of antimicrobial therapy are to treat acute infection, relieve symptoms and prevent relapse. The agents often used in the treatment of BSD are rifampicin, doxycycline, trimethoprim/sulfamethoxazole, ciprofloxacin, gentamicin and streptomycin [42]. Antibiotic treatment must combine at least two active antibiotics in order to reduce the rate of therapeutic failure and relapse when treatment is stopped [43]. The World Health Organization recommend the use of doxycycline (100 mg twice a day) plus rifampin (600 mg/day) plus streptomycin (1 g per day for 21 days). After 3 weeks, patients continue treatment with doxycycline plus rifampin over six months [44]. In our study, treatment duration were longer, which might lead to the increase of adverse effects and possibly the emergence of antibiotic resistance. Along with medical treatment, surgical procedure might be required for a specific patients with spinal abscess, vertebral collapse, bone destruction and cord compression [42].
To conclude, BSD remain a public health concern in endemic countries warranting the implementation of an urgent strategy for the eradication of animal brucellosis and the implementation of food safety and hygiene measures for human prevention.
References
- Shakir R. Brucellosis. J Neurol Sci. 2021; 420: 117280.
- Akhvlediani T, Clark DV, Chubabria G, Zenaishvili O, Hepburn MJ. The changing pattern of human brucellosis: Clinical manifestations, epidemiology, and treatment outcomes over three decades in Georgia. BMC Infect Dis. 2010; 10: 346.
- Galińska EM, Zagórski J. Brucellosis in humans-etiology, diagnostics, clinical forms. Ann Agric Environ Med AAEM. 2013; 20: 233‑8.
- Identification of Brucella spp. isolated from human brucellosis in Malaysia using High-Resolution Melt (HRM) analysis. Diagn Microbiol Infect Dis. 2015; 81: 227‑33.
- Harrison ER, Posada R. Brucellosis. Pediatr Rev. 2018; 39: 222‑4.
- Assenga JA, Matemba LE, Muller SK, Malakalinga JJ, Kazwala RR. Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi-Rukwa ecosystem, Tanzania. BMC Vet Res. 2015; 11: 189.
- Turgut M, Turgut AT, Koşar U. Spinal brucellosis: Turkish experience based on 452 cases published during the last century. Acta Neurochir (Wien). 2006; 148: 1033‑44.
- Harman M, Unal O, Onbaşi KT, Kiymaz N, Arslan H. Brucellar spondylodiscitis: MRI diagnosis. Clin Imaging. 2001; 25: 421‑7.
- Tali ET, Koc AM, Oner AY. Spinal brucellosis. Neuroimaging Clin N Am. 2015; 25: 233‑45.
- J Solera, E Lozano, E Martínez-Alfaro, A Espinosa, ML Castillejos, et al. Brucellar spondylitis: Review of 35 cases and literature survey. Clin Infect Dis. 1999; 29: 1440-9.
- Kaptan F, Gulduren HM, Sarsilmaz A, Sucu HK, Ural S, et al. Brucellar spondylodiscitis: Comparison of patients with and without abscesses. Rheumatol Int. 2013; 33: 985‑92.
- Maurin M. Brucellosis at the dawn of the 21st century. Med Mal Infect. 2005; 35: 6‑16.
- Mehmet Faruk Geyik, Ali Gür, Kemal Nas, Remzi Cevik, Jale Saraç, Bunyamin Dikici, et al. Musculoskeletal involvement of brucellosis in different age groups: A study of 195 cases. Swiss Med Wkly. 2002; 132: 98-105.
- Zribi M, Ammari L, Masmoudi A, Tiouiri H, Fendri C. Aspects cliniques, microbiologiques et thérapeutiques de la brucellose: Etude de 45 cas. Pathol Biol. 2009; 57: 349‑52.
- Lounes N, Cherfa MA, Carrou GL, Bouyoucef A, Jay M, et al. Human Brucellosis in Maghreb: Existence of a Lineage Related to Socio-Historical Connections with Europe. PLOS ONE. 2014; 9: e115319.
- Buzgan T, Karahocagil MK, Irmak H, Baran AI, Karsen H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2010; 14: e469-478.
- Ugarriza LF, Porras LF, Lorenzana LM, Rodríguez-Sánchez JA, García-Yagüe LM, et al. Brucellar spinal epidural abscesses. Analysis of eleven cases. Br J Neurosurg. 2005; 19: 235‑40.
- Mohamed Refai. Incidence and control of brucellosis in the Near East region. Vet Microbiol. 2002; 90: 81-110.
- Mile Bosilkovski, Ljiljana Krteva, Marija Dimzova, Irena Kondova. Brucellosis in 418 patients from the Balkan Peninsula: exposurerelated differences in clinical manifestations, laboratory test results, and therapy outcome. Int J Infect Dis. 2007; 11: 342-7.
- Seyed Mokhtar Smailnejad Gangi, Mohammad Reza Hasanjani Roushan, Nasser Janmohammadi, Raheleh Mehraeen, Mohammad Jafar Soleimani Amiri, et al. Outcomes of treatment in 50 cases with spinal brucellosis in Babol, Northern Iran. J Infect Dev Ctries. 2012; 6: 654-9.
- Selma Guler, Omer Faruk Kokoglu, Hasan Ucmak, Mustafa Gul, Sevinc Ozden, et al. Human brucellosis in Turkey: Different clinical presentations. J Infect Dev Ctries. 2014; 8: 581-8.
- Panos Andriopoulos, Maria Tsironi, Spiros Deftereos, Athanassios Aessopos, Giorgos Assimakopoulos. Acute brucellosis: Presentation, diagnosis, and treatment of 144 cases. Int J Infect Dis. 2007; 11: 52-7.
- Mouna Chelli Bouaziz, Mohamed Fethi Ladeb, Mohamed Chakroun, Skander Chaabane. Spinal brucellosis: A review. Skeletal Radiol. 2008; 37: 785-90.
- Mehmet Aydin, A Fuat Yapar, Lutfu Savas, Mehmet Reyhan, Aysin Pourbagher, et al. Scintigraphic findings in osteoarticular brucellosis. Nucl Med Commun. 2005; 26: 639-47.
- Mile Bosilkovski, Ljiljana Krteva, Sonja Caparoska, Marija Dimzova. Osteoarticular involvement in brucellosis: study of 196 cases in the Republic of Macedonia. Croat Med J. 2004; 45: 727-33.
- Tuba Turunc, Yusuf Ziya Demiroglu, Hikmet Uncu, Sule Colakoglu, Hande Arslan. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007; 55: 158-63.
- Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, Reguera JM, Palomino-Nicás J, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997; 56: 709‑15.
- Oztekin O, Calli C, Adibelli Z, Kitis O, Eren C, et al. Brucellar spondylodiscitis: magnetic resonance imaging features with conventional sequences and diffusion-weighted imaging. Radiol Med (Torino). 2010; 115: 794‑803.
- Sans N, Faruch M, Lapègue F, Ponsot A, Chiavassa H, et al. Infections of the spinal column – Spondylodiscitis. Diagn Interv Imaging. 2012; 93: 520‑9.
- L Grammatico, S Baron, E Rusch, B Lepage, N Surer, et al. Epidemiology of Vertebral Osteomyelitis (VO) in France: Analysis of hospital-discharge data 2002-2003. Epidemiol Infect. 2008; 136: 653-660.
- Mustafa Namiduru, Ilkay Karaoglan, Savas Gursoy, Nurhayat Bayazit, Akif Sirikci. Brucellosis of the spine: evaluation of the clinical, laboratory, and radiological findings of 14 patients. Rheumatol Int. 2004; 24: 125-9.
- Ebru Kursun, Tuba Turunc, Yusuf Demiroglu, Hande Arslan. Evaluation of four hundred and forty seven brucellosis cases. Intern Med. 2013; 52: 745-50.
- Aysin Pourbagher, Mir Ali Pourbagher, Lutfu Savas, Tuba Turunc, Yusuf Ziya Demiroglu, et al. Epidemiologic, clinical, and imaging findings in brucellosis patients with osteoarticular involvement. AJR Am J Roentgenol. 2006; 187: 873-80.
- Bozgeyik Z, Ozdemir H, Demirdag K, Ozden M, Sonmezgoz F, et al. Clinical and MRI findings of brucellar spondylodiscitis. Eur J Radiol. 2008; 67: 153‑8.
- Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, et al. Clinical manifestations of human brucellosis: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2012; 6: e1929.
- M Chakroun, N Bouzouaia. La brucellose: Une zoonose toujours d’actualité. Brucellosis: A topical zoonosis. Rev Tun Infectiol. 7; 1: 1-10.
- JP Lavigne, A Mailles, A Sotto. Brucellose-EM consulte. 8-038-A10.
- Yagupsky P, Morata P, Colmenero JD. Laboratory Diagnosis of Human Brucellosis. Clin Microbiol Rev. 2019; 33: e00073-19.
- Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: A review. World J Orthop. 2019; 10: 54‑62.
- Hammami F, Koubaa M, Feki W, Chakroun A, Rekik K, et al. Tuberculous and Brucellar Spondylodiscitis: Comparative Analysis of Clinical, Laboratory, and Radiological Features. Asian Spine J. 2021; 15: 739‑46.
- Masson E. Imagerie des spondylodiscites infectieuses. EM-Consulte. 31-335-A-10.
- Unuvar GK, Kilic AU, Doganay M. Current therapeutic strategy in osteoarticular brucellosis. North Clin Istanb. 2019; 6: 415‑20.
- Ulu-Kilic A, Karakas A, Erdem H, Turker T, Inal AS, et al. Update on treatment options for spinal brucellosis. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2014; 20: O75-82.
- WHO, Nations F and AO of the U, Organization WH, Health WO for A. Brucellosis in humans and animals. 2006. Report No: WHO/CDS/EPR/2006.7.