SciBase Journals
SciBase Clinical and Medical Case Reports
ISSN 2995-5874
- Article Type: Research Article
- Volume 2, Issue 1
- Received: Jan 17, 2024
- Accepted: Feb 19, 2024
- Published Online: Feb 26, 2024
Application Value of Serum Cytokines in Respiratory Syncytial Virus Pneumonia in Children
Xinxin Wang; Jiahao Geng; Baowen Li; Shujun Li*
Department of Pediatrics, The First Affiliated Hospital of Xinxiang Medical College, No. 88, Health Road, Xinxiang City, 453100, Henan Province, China.
*Corresponding Author: Shujun Li
Department of Pediatrics, The First Affiliated Hospital of Xinxiang Medical College, No. 88, Health Road, Xinxiang City, 453100, Henan Province, China.
Email: picu3390@126.com
Abstract
Objective: To analyze the relationship between cytokines and clinical manifestations of Respiratory Syncytial Virus (RSV) infection and to investigate the clinical value of cytokines in RSV pneumonia.
Methods: Clinical data of RSV positive children admitted to the pediatrics department of Zhengzhou People’s Hospital from November 2021 to June 2023. They were divided into three groups according to different ages, 1-11 months, 12-23 months, 24-36 months, and wheezing group and no wheezing group according to the presence of complications. The clinical data of the children in different groups were compared, and the clinical significance of the children with RSV pneumonia was analyzed by measuring the serum cytokine levels.
Results: A total of 64 children were included, 35 from 1-11,16 from 12-23 and 13 in 24-36. There were 35 patients with wheezing and 29 patients with no wheezing. Complications were found in 6 cases and 58 cases were without complications. SPO2 , N and CRP in the 1-November group were lower than those in the 12-2 March group, L was higher than that in the 12-23-month group, the proportion of severe, oxygen inhalation, three concave sign, R, P, IL-8, L were higher than those in the 23-36 month group, Oxygen Saturation (SPO2 ), C-Reactive Proteins (CRP), Procalcitonin (PCT) below the 23-3 June group, the difference is statistically significant; 12-23 month group: CDSS, R, IL-8 were higher than the 24-36 month group. The PCT was lower than that in the 24-3 June group. The difference was statistically significant (P<0.05). Clinical characteristics and cytokine analysis of patients with wheezing group and no wheezing group: the fever days, IL-2, and lymphocyte count were higher in patients without wheezing, with statistically significant differences (P<0.05). In terms of length of stay, R, P, IL-1 β, IL-6, and IL-8, patients with complications were higher than patients without complications, with a statistically significant difference (P<0.05).
Conclusion: IL-1β, IL-2, IL-4, IL-6, IL-8 and IL-10 are related with different clinical symptoms and have certain guiding significance for clinical application. IL-1β, IL-2, IL-4, IL-6, IL-8 and IL-10 have different expression levels. IL-2 may be related with wheezing, and IL-1β, IL-6 and IL-8 may be related to complications, which has certain guiding significance for clinical application.
Keywords: Respiratory syncytial virus; Cytokines; Clinical features; Application value; Children.
Abbreviations: RSV: Respiratory Syncytial Virus; CRP: C-Reactive Protein; SPO2 : Oxygen Saturation; PCT: Procalcitonin; IL-1β: Interleukin-1β; L-2: Interleukin-2; IL-4: Interleukin-4; IL-6: Interleukin-6; IL-8: Interleukin-8; IL-10: Interleukin-10.
Citation: Wang X, Geng J, Li B, Li S. Application Value of Serum Cytokines in Respiratory Syncytial Virus Pneumonia in Children. SciBase Clin Med Case Rep. 2024; 2(1): 1015.
Introduction
RSV infection is the most important cause of lower respiratory tract infections (Lower Respiratory Tract Infections, LRTI) in children worldwide. According to statistics, RSV infections accounted for 22% of all pediatric acute respiratory infections in the world in 2005, with 10% of the patients requiring hospitalization and approximately 55000 to 199000 children dying [1]. The latest statistics show that more than 95% of RSV-associated acute lower respiratory tract infections and more than 97% of RSV-induced deaths in 2015-2019 occurred in low-and middleincome countries, slightly lower than before [2]. In China, according to survey research, RSV infection rate is high, but RSV monitoring network has not been established in China [3].
The pathogenesis of pneumonia due to RSV is not fully understood. Studies have shown that most of the airway damage by RSV is mediated by the immune response and not directly damaged by the virus. After RSV infection, patients infiltrate immune cells such as chemotaxis and neutrophils, they develop mucosal edema, cause airway contracture, and eventually pneumonia and other diseases [4]. RSV infection, by mediating innate and adaptive immune responses, leads to the production of proinflammatory cytokines and chemokines, such as IFN- γ, IL-13, IL-17, IL-5 and so on, and recruits inflammatory cells to the airways, such as Dendritic Cells (DC), neutrophils, monocytes, lymphocytes, macrophages, etc [5], lead to mucosal edema, causing airway stenosis, and eventually appear bronchiolitis, pneumonia and other diseases [6]. At present, there are few studies on the expression levels of serum cytokines IL-1 β, IL-2, IL-4, IL-6, IL-6, IL-8 and IL-10 and clinical characteristics of children. This study will explore the clinical value of serum cytokines in RSV pneumonia by measuring the level of RSV pneumonia.
Materials and methods
Normal information
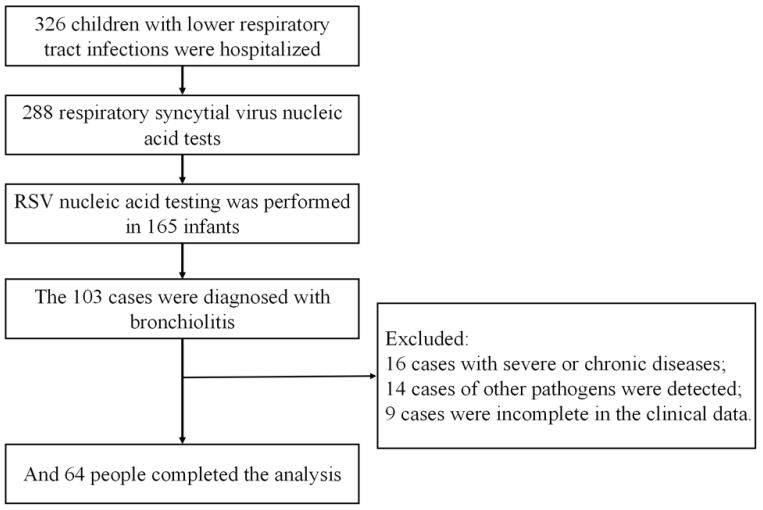
Children with RSV pneumonia admitted to Zhengzhou People’s Hospital from November 2021 to June 2023 were collected for a case-control study. This study was approved by the hospital ethics committee (approval number: 2021L12011), and all the parents or guardians of the participating patients gave the informed consent and signed the informed consent form.
Inclusion criteria: (1) Age 29 days to 36 months; (2) RSV bronchobronchial pneumonia diagnostic criteria met the 2019 Respiratory syncytial virus: diagnosis, prevention and management diagnostic criteria [7]: Nasal catarras symptoms, cough, wheezing, dyspnea, cyanosis, signs such as breathing rate, expiratory phase extension, smell and wheezing and fine rales, X-ray for pulmonary hyperinflation or patchy shadow, local atelectasis, peribronchial inflammation, and simultaneous pharyngeal swab PCR suggests positive RSV-DNA.
Exclusion criteria: (1) Severe or chronic diseases (such as congenital heart disease, chronic lung disease, congenital or acquired immune deficiency, etc.); (2) abnormal development of weather channels or lung structures; (3) use of steroid hormones in the last two weeks; (4) premature infants; (5) pneumonia induced by foreign bodies; (6) incomplete medical records; (7) detection of other pathogens.
Research methods
Collection included clinical data of children: (1) demographic data such as age, sex, weight, length of stay; (2) pre-hospital course, cough, fever, wheezing, admission signs including heart rate, respiration, temperature, oxygen saturation, and three concave symptoms; (3) auxiliary examination: white blood cell count, neutrophil count, lymphocyte count, Lactate Dehydrogenase (LDH), and Procalcitonin (PCT); (4) chest imaging.
Group
Month-age group: According to a multicenter study of RSV in Hubei Province [8] Group, 1-1 group (35), 12-23 group (16), and 24-36 group (13).
Group of wheezing: According to the presence of first wheezing symptoms on admission [9]: 35 patients had wheezing, and 29 patients had no wheezing.
Group of complications: According to the presence of convulsions, myocardial damage, pleural effusion, electrolyte disorder, respiratory failure and other complications, there were 6 cases with complications and 58 cases without complications.
Retention and treatment of specimens
All the children had the first wheezing attack, which were collected on an empty stomach in the morning of the next day after admission. 2 ml of venous blood was collected at room temperature. Blood was centrifuged for 10 minutes (4000 rpm), and 0.5 ml of serum solution was carefully collected and stored in 2 to 6OC refrigerator for testing. The levels of serum IL-1 β, IL-2, IL-4, IL-6, IL-8 and IL-10 were measured by using LUMINEX® 100 (Luminex Corporation, USA) using flow immunofluorescence kit (Nanjing Alto Life Technology Co., LTD., Batch No.21010401).
Statistical method
Statistics were performed using the SPSS26.0 software. Continuous variables were tested for normality, normally distributed variables were represented by the mean ± standard deviation and the independent-sample t-test was analyzed; non-normally distributed variables were represented by the median (interquartile spacing), and Mann-Whitney U test or rank-sum test compared the differences between groups. Categorical variables were expressed as percentages, and differences between groups were compared using chi-square, continuous correction or Fisher’s exact test.
Results
Comparison of clinical characteristics and laboratory examination of each age group: severe and oxygen inhalation ratio, body weight, IL-8, neutrophil count (N), lymphocyte count (L), CRP, PCT, CDSS score, SPO2, and fever time between the three age groups; Pairwise comparisons of each age group: SPO2, N, CRP in 1-1 than 12-23, L was higher than that in the 12-23-month group, The proportion of severe, oxygen inhalation, three concave sign, IL-8 and L were higher than those in the 23-36 group, SPO2, CRP and PCT were lower than the group of 23-36 months; CDSS and IL-8 were higher in the 12-23 group than in the 24- 36 group, The PCT was lower than that in the 24-3 June group; P<0.05, Table 1.
Clinical characteristics and cytokines clinical characteristics and cytokine analysis of patients in wheeze and no wheezing: high fever days, IL-2, lymphocyte count, and statistically significant differences (P<0.05) (Table 2).
Table 1: Frontal CT scan showing the mass an upper polar tissue mass of the left kidney.
| Variable | January-November (n=35) | 12-2 March (n=16) | 24-3 June (n=13) | Z/χ2 | P* |
|---|---|---|---|---|---|
| Gender, male, n(%) | 22(62.86) | 11(68.75) | 8(61.54) | 0.211 | 0.900 |
| severe, n(%) | 13(37.14) | 2(12.5) | 0 | 8.710 | 0.013* |
| weight (kg) | 8.6(7.15,10)◇△ | 11(10,12.10)△ | 12(11,13)△ | 22.06 | 0.00* |
| Prehospital course (days) | 4(3,5) | 3(2,4.25) | 4(3,5) | 2.37 | 0.31 |
| Cough (day) | 3(2,5) | 3(2,3) | 4(3,5) | 1.51 | 0.47 |
| Fever (day) | 0(0,1.5)△ | 0.75(0.125,3.25) | 3(2,4)△ | 10.05 | 0.01* |
| Breathing (day) | 1(0,2.5) | 0.75(0,1.25) | 0(0,2) | 1.79 | 0.41 |
| Length of hospital stay (day) | 7(5.5,7) | 6.5(5,9) | 6(5,8) | 0.26 | 0.88 |
| CDSS grade | 3(2,6)△ | 3(2,4)# | 2(1,2)#△ | 10.70 | 0.00* |
| T(OC) | 36.8(36.55,37.25) | 37(36.7,37.53) | 36.9(36.5,37.6) | 0.93 | 0.63 |
| R (sub/cent) | 35(30,39.5)△ | 31.5(30,36.5)# | 28(28,30)#△ | 12.36 | 0.00* |
| P (sub/cent) | 136(126,145.5)△ | 128(124.75,138.5) | 125(120,128)△ | 10.85 | 0.00* |
| SPO2(%) | 96(93,98)◇△ | 98(96,98)◇ | 98(98,98)△ | 17.04 | 0.00* |
| oxygen uptake, n(%) | 13(37.14)△ | 4(25) | 0△ | 6.731 | 0.035* |
| Three-concave sign, n(%) | 18(51.43)△ | 8(50) | 2(15.38)△ | 5.343 | 0.069 |
| Complications, and the n(%) | 14(11.43) | 1(6.25) | 1(7.69) | 0.401 | 0.818 |
| IL-1β(pg/mL) | 10.59(3,33.25) | 10.3(4.38,23.55) | 7.8(3.3,12.5) | 0.70 | 0.71 |
| IL-2(pg/mL) | 7.6(4,31.06) | 10.3(4,48.13) | 29.6(4,39.4) | 1.08 | 0.58 |
| IL-4(pg/mL) | 8.02(3,33.5) | 5.65(3.10,8.53) | 4.3(3,25.1) | 1.27 | 0.53 |
| IL-6(pg/mL) | 8.8(2,19.23) | 6.4(2.4,17.45) | 5.4(2,14.3) | 0.24 | 0.89 |
| IL-8(pg/mL) | 5.6(3,21.55)△ | 3.85(3,8.78)# | 3(3,3)#△ | 7.10 | 0.03* |
| IL-10(pg/mL) | 13.36(3.7,32.75) | 9.75(4.82,22.73) | 7.3(3.9,15.3) | 0.67 | 0.72 |
| WBC(×109/L) | 9.5(7.58,11.57) | 8.85(6.88,11.99) | 6.49(5.76,9.74) | 3.34 | 0.19 |
| N(×109/L) | 2.28(1.73,3.3)△ | 3.55(3.04,4.19)△ | 3.22(1.92,5.1) | 8.74 | 0.01* |
| L(×109/L) | 6.22(4.67,7.63)◇△ | 4.01(2.53,6.29)△ | 3(2.3,3.64)△ | 17.58 | 0.00* |
| CRP(mg/L) | 2.67(0.85,4.22)◇△ | 4.2(3.59,5.54)△ | 4.3(3.84,7.98)△ | 14.72 | 0.00* |
| PCT(ng/ml) | 0.06(0.04,0.08)△ | 0.07(0.06, 0.08)# | 0.1(0.08,0.14)#△ | 16.34 | 0.00* |
| LDH(U/L) | 338(290,398.5) | 358(296.75,415) | 327(280,366) | 1.16 | 0.56 |
Note: *: The difference between the three groups; ◇: The difference between the 1-November group and the 12-2 March group and the 24-3 June group; △: The difference between the 1-November group and the 23-3 June group; P<0.05 indicates the difference between the two groups.
Table 2: Clinical characteristics and cytokine analysis of the wheezing group and no wheezing patients.
| Variable | No wheezing (n=29) | Respite group (n=35) | Z/χ2 | P |
|---|---|---|---|---|
| Sex, male, and n(%) | 17(58.62) | 24(68.57) | 0.682 | 0.409 |
| Age (month) | 9(1.5,24) | 8(6,12) | 0.456 | 0.649 |
| weight (kg) | 10(7.4,12.7) | 10(8.5,11) | 0.460 | 0.645 |
| severe, n(%) | 7(24.14) | 8(22.86) | 0.014 | 0.904 |
| CDSS grade | 3(1,5.5) | 3(2,5) | 0.759 | 0.448 |
| Prehospital course (days) | 4(2,5) | 4(3,5) | 0.082 | 0.934 |
| Cough (day) | 4(2,5) | 3(3,4) | 0.659 | 0.510 |
| Fever (day) | 3(0,5) | 0.125(0,2) | 2.962 | 0.003 |
| Breathing (day) | 7(5,9) | 6(5,8) | 1.116 | 0.264 |
| T(OC) | 37.1(36.5,37.9) | 36.8(36.6,37.1) | 1.555 | 0.120 |
| R (sub/cent) | 32(30,38) | 31(28,38) | 0.354 | 0.724 |
| P (sub/cent) | 129(125,143) | 129(123,140) | 0.392 | 0.695 |
| SPO2(%) | 98(34.5,98) | 98(96,98) | 0.440 | 0.660 |
| oxygen uptake, n(%) | 8(27.59) | 9(25.71) | 0.028 | 0.886 |
| Three-concave sign, n(%) | 12(41.38) | 16(45.71) | 0.121 | 0.728 |
| Complications, and the n(%) | 3(10.34) | 3(8.57) | 0.059 | 0.809 |
| IL-1β(pg/mL) | 9.3(3,25.9) | 9.7(3,32.2) | 0.560 | 0.575 |
| IL-2(pg/mL) | 4.1(4,31.9) | 24.9(6.2,46) | 2.424 | 0.015 |
| IL-4(pg/mL) | 4.3(3,28.1) | 8.4(3,25.7) | 0.870 | 0.384 |
| IL-6(pg/mL) | 9.7(2,23.32) | 5(2,17) | 1.230 | 0.219 |
| IL-8(pg/mL) | 3(3,12.9) | 4.99(3,14) | 1.110 | 0.267 |
| IL-10(pg/mL) | 4.93(3.5,22.7) | 15.1(4.5,34) | 1.351 | 0.177 |
| WBC (×109/L) | 8.69(6.10,10.12) | 9.63(7.17,12.48) | 1.639 | 0.101 |
| N(×109/L) | 2.41(1.9,3.65) | 2.74(1.88,4.15) | 0.533 | 0.594 |
| L(×109/L) | 4.58(2.67,5.78) | 6.13(3.98,7.71) | 2.030 | 0.042 |
| CRP(mg/L) | 3.84(2.21,5.21) | 3.54(1.64,5.11) | 0.331 | 0.741 |
| PCT(ng/ml) | 0.078(0.045,0.11) | 0.067(0.048,0.081) | 1.261 | 0.207 |
| LDH(U/L) | 327(285,381.5) | 350(296,415) | 1.308 | 0.191 |
Note: P<0.05 indicates a statistically significant difference between the two groups.
Table 3: Compares the clinical data with the cytokines.
| Variable | Uncomplicated group (n=58) | Group with complications (n=6) | Z/χ2 | P |
|---|---|---|---|---|
| Sex, male, and n (%) | 37(63.79) | 4(66.67) | 0.02 | 0.899 |
| Age (month) | 9.00(5.00,13.50) | 4.50(1.75,16.75) | 0.813 | 0.416 |
| weight (kg) | 10.00(8.22,12.00) | 9.40(7.72,10.25) | 1.075 | 0.283 |
| severe, n(%) | 11(18.97) | 4(66.67) | 6.895 | 0.09 |
| CDSS grade | 3.00(2.00,5.00) | 6.00(1.00,6.00) | 0.935 | 0.350 |
| Prehospital course (days) | 4.00(3.00,5.00) | 3.50(1.62,5.50) | 0.457 | 0.648 |
| Cough (day) | 3.00(2.00,5.00) | 3.50(1.50,5.50) | 0.000 | 1.000 |
| Fever (day) | 1.00(0.00,3.25) | 0.25(0.00,3.25) | 0.548 | 0.584 |
| Breathing (day) | 0.75(0.00,2.00) | 0.50(0.00,3.25) | 0.000 | 1.000 |
| Length of hospital stay (days) | 6.00(5.00,8.00) | 8.50(6.75,9.25) | 2.023 | 0.043 |
| T(OC) | 36.80(36.50,37.33) | 37.15(36.98,38.60) | 1.766 | 0.077 |
| R (sub/cent) | 30.00(28.00,37.25) | 40.00(33.75,42.75) | 2.311 | 0.021 |
| P (sub/cent) | 128.50(123.75,139.25) | 146.00(137.00,150.00) | 2.341 | 0.019 |
| SPO2(%) | 98.00(96.00,98.00) | 93.50(92.00,98.00) | 1.808 | 0.071 |
| oxygen uptake, n(%) | 14(24.14) | 3(50) | 1.864 | 0.172 |
| Three-concave sign, n(%) | 24(41.38) | 4(66.67) | 1.413 | 0.235 |
| Complications, and the n(%) | 7.70(3.00,28.02) | 27.80(18.07,103.28) | 2.532 | 0.011 |
| IL-1β(pg/mL) | 15.41(4.00,43.40) | 4.70(3.98,9.18) | 1.895 | 0.058 |
| IL-2(pg/mL) | 6.40(3.00,23.04) | 36.20(4.80,300.55) | 1.848 | 0.065 |
| IL-4(pg/mL) | 5.20(2.00,17.07) | 20.35(17.16,22.45) | 2.334 | 0.020 |
| IL-6(pg/mL) | 3.00(3.00,11.72) | 37.20(6.50,55.58) | 3.009 | 0.003 |
| IL-8(pg/mL) | 8.70(3.80,26.31) | 33.30(7.85,76.70) | 1.684 | 0.092 |
| IL-10(pg/mL) | 8.85(6.80,10.74) | 11.98(8.55,13.52) | 1.543 | 0.123 |
| WBC(×109/L) | 2.70(1.88,3.83) | 2.38(1.71,8.67) | 0.058 | 0.954 |
| N(×109/L) | 4.87(3.05,6.99) | 5.62(1.91,10.30) | 0.138 | 0.890 |
| L(×109/L) | 3.57(1.89,5.21) | 4.31(2.90,11.87) | 1.002 | 0.316 |
| CRP(mg/L) | 0.07(0.05,0.09) | 0.07(0.05,0.75) | 0.230 | 0.818 |
| PCT(ng/ml) | 340.00(285.00,399.50) | 317.00(271.50,378.25) | 0.806 | 0.420 |
Note: P>0.05 indicates no significant difference between the two groups, and P<0.05 indicates a significant difference between the two groups.
Clinical characteristics and cytokine analysis of patients with complications compared with and without complications: length of stay, R, P, IL-1 β, IL-6, and IL-8, patients with complications than those without complications showed statistically significant differences (P<0.05), as shown in Table 3.
Discussion
In the 1850s, respiratory syncytial virus (Respiratory Syncytial Virus, RSV) was isolated from infants and mammals [11]. It has a wide impact on people of all ages. Since 1986, the most important pathogen of severe lower respiratory tract infections (Lower Respiratory Tract Infections, LRTI) in children worldwide is RSV [12]. From the summary of a number of retrospective studies in China, it can be concluded that the RSV positive rate is 11.1%-14.3%, and the detection rate of infants under 1 year old is 15.9%-21.6%. The high incidence season is from December to February of the next year, the median age is 13 months, and the ratio of male to female is 1.8:1 [8,13], 8% of patients with RSV infection progressed to severe patients [14]. Fifty percent of patients with RSV are infected twice under 2 years of age [15]. Even repeated infections [16]. In this study, 35 patients, 54.69%, 16 patients in 12-2 March, and 13 patients in 24-3 June, 20.31%. From January to November, the proportion of patients with low SPO2 , severe severity and three concave sign was higher than those of other age patients, which was statistically significant.
Patients started developing respiratory symptoms about 3 to 7 days after RSV infection [17], can lead to hospitalization, serious complications, sequelae and even death [14]. The RSV susceptibility population is reserved for preterm birth, combined congenital heart disease, bronchopulmonary dysplasia, genetic metabolic diseases, and neuromuscular diseases [18]. RSV mainly involves the respiratory tract, and researchers have also isolated RSV or its genetic material from cerebrospinal fluid, cardiac muscle, liver, and peripheral blood [19]. This shows that RSV can involve multiple organs, causing severe arrhythmias such as supraventricular tachycardia and ventricular tachycardia, central apnea, focal and systemic seizures, hyponatremia, antidiuretic hormone secretion and hepatitis, and even death [20-22]. In this study, serum cytokines IL-1 β, IL-1, IL-6 and IL-8 were higher in complicated patients than uncomplicated patients, and complications were mainly tics and myocardial damage.
The infectivity, disease severity and degree of cellular pathological changes of RSV are directly related to viral load as well as structural specificity [23]. The structural proteins of RSV evade host immunity through multiple mechanisms. NS1 and NS2 proteins inhibit type I IFN release from epithelial cells through the JAK/STAT pathway or toll-like receptor, disrupting T lymphocyte activation and dendritic cell recruitment [24]. Furthermore, NS1 and NS2 proteins also reduce apoptosis through activation of the PI3k pathway to prolong the survival of infected cells, eventually leading to prolonged viral replication [25]. Alternatively, G proteins are also able to limit leukocyte recruitment, induce antibody neutralization and delay antigen recognition [26]. RSV infection, by mediating innate and adaptive immune responses, leads to pro-inflammatory cytokines and chemokine-production, such as IFN- γ, IFL-13, IL-17, IL-8, IL-5, and IL-4, and recruits inflammatory cells to the airways, such as Dendritic Cells (DC), neutrophils, monocytes, lymphocytes, macrophages, etc [27]. Lead to mucosal edema, causing airway stenosis, and eventually appear bronchiolitis, pneumonia and other diseases [28].
This study showed that IL-2 and lymphocyte counts were higher in RSV pneumonia patients compared with patients without wheezing. Some researchers have found higher concentrations of IL-2, TNF- α, IL-10 and IL-17 than those in children with no wheezing [9]. Consistent with this study. IL-2 is a cytokine critical for the growth, survival, and function of CD4 + T cells, especially FOXP 3 + regulatory T cells (Tregs), while disease severity was found to be inversely correlated with the ability of CD4 + T cells to produce IL-2 and the ability of exogenous IL-2 to restore the FOXP 3 + CD4 + T cell pool [29]. RSV infection can affect the production and function of IL-2 and Tregs, leading to an impaired immune response and increased disease severity [30]. This study has some limitations, including a small sample size and some bias in the results of each index. This study is a single-center study and can be verified by a multi-center joint study in the future.
Conclusion
IL-1 β, IL-2, IL-4, IL-6, IL-8, IL-10 are related with different clinical symptoms, and have certain guiding significance for clinical application. IL-1 β, IL-2, IL-4, IL-6, IL-8 and IL-10 have different expression levels. IL-2 may be related with wheezing, and IL-1 β, IL-6 and IL-8 may be related to complications, which has certain guiding significance for clinical application.
Declarations
Authors’ contributions: Conception and design of the work: WXX; Data collection: WXX; Supervision: WXX; Analysis and interpretation of the data: WXX; Statistical analysis: WXX; Drafting the manuscript: WXX; Critical revision of the manuscript: WXX, LSJ; Approval of the final manuscript: all authors.
Data availability: All data generated or analyzed during this study are included in this published article.
Acknowledgement: Ethics approval and consent to participate. This study was conducted in accordance with the declaration of Helsinki and approved by the Medical Ethics Committee of Zhengzhou People’s Hospital (2021L12011), and with informed consent from the guardians.
Competing interests: All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
- Nair H, Nokes D J, Gessner B D, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010; 375(9725): 1545-55.
- Li Y, Wang X, Blau D M, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340): 2047-64.
- Scheltema N M, Gentile A, Lucion F, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017; 5(10): e984-e91.
- Norlander A E, Peebles R S, Jr. Innate Type 2 Responses to Respiratory Syncytial Virus Infection. Viruses. 2020; 12(5).
- De C, Pickles R J, Yao W, et al. Human T cells efficiently control RSV infection. JCI insight. 2023; 8(11).
- Santos L D, Antunes K H, Muraro S P, et al. TNF-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection. The European respiratory journal. 2021; 57(6).
- Barr R, Green CA, Sande CJ, Drysdale SB. Respiratory syncytial virus: diagnosis, prevention and management. Ther Adv Infect Dis. 2019; 6: 2049936119865798.
- Hu X W, Zhou Y, Yi S, et al. Epidemiological Characteristics of Respiratory Syncytial Virus Infection Among Hospitalized Children With Acute Respiratory Tract Infections From 2014 to 2022 in a Hospital in Hubei Province, China: Longitudinal Surveillance Study. JMIR Public Health Surveill. 2023; 9: e43941.
- Rodriguez-Fernandez R, Gonzalez-Martinez F, Gonzalez-Sanchez M I, et al. Longitudinal plasma cytokine concentrations and recurrent wheezing after RSV bronchiolitis. Cytokine. 2021; 140: 155434.
- Garcia-Maurino C, Moore-Clingenpeel M, Thomas J, et al. Viral Load Dynamics and Clinical Disease Severity in Infants With Respiratory Syncytial Virus Infection. J Infect Dis. 2019; 219(8): 1207-15.
- Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957; 66(3): 281-90.
- Rima B, Collins P, Easton A, et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J Gen Virol. 2017; 98(12): 2912-3.
- Binns E, Tuckerman J, Licciardi P V, et al. Respiratory syncytial virus, recurrent wheeze and asthma: A narrative review of pathophysiology, prevention and future directions. J Paediatr Child Health. 2022; 58(10): 1741-6.
- Yu J, Liu C, Xiao Y, et al. Respiratory Syncytial Virus Seasonality, Beijing, China, 2007-2015. Emerg Infect Dis. 2019; 25(6): 1127-35.
- Glezen W P, Taber L H, Frank A L, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986; 140(6): 543-6.
- Taleb S A, Al Thani A A, Al Ansari K, et al. Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2018; 37(10): 1817-27.
- Nam H H, Ison M G. Respiratory syncytial virus infection in adults. BMJ. 2019; 366: l5021.
- Scheltema N M, Gentile A, Lucion F, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017; 5(10): e984-e91.
- Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection--a systematic review. Crit Care. 2006; 10(4): R107.
- Bottino P, Miglino R, Pastrone L, et al. Clinical features of respiratory syncytial virus bronchiolitis in an infant: rapid and fatal brain involvement. BMC Pediatr. 2021; 21(1): 556.
- Xu L, Gao H, Zeng J, et al. A fatal case associated with respiratory syncytial virus infection in a young child. BMC Infect Dis. 2018; 18(1): 217.
- Domachowske J B, Anderson E J, Goldstein M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect Dis Ther. 2021; 10(Suppl 1): 47-60.
- Talukdar S N, McGregor B, Osan J K, et al. Respiratory Syncytial Virus Infection Does Not Induce Epithelial-Mesenchymal Transition. Journal of virology. 2023; 97(7): e0039423.
- Kampmann B, Madhi S A, Munjal I, et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. The New England journal of medicine. 2023; 388(16): 1451-64.
- Van Royen T, Rossey I, Sedeyn K, et al. How RSV Proteins Join Forces to Overcome the Host Innate Immune Response. Viruses. 2022; 14(2).
- Bergeron H C, Kauvar L M, Tripp R A. Anti-G protein antibodies targeting the RSV G protein CX3C chemokine region improve the interferon response. Ther Adv Infect Dis. 2023; 10: 20499361231161157.
- De C, Pickles R J, Yao W, et al. Human T cells efficiently control RSV infection. JCI insight. 2023; 8(11).
- Santos L D, Antunes K H, Muraro S P, et al. TNF-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection. The European respiratory journal. 2021; 57(6).
- Sananez I, Raiden S, Erra-Diaz F, et al. Dampening of IL-2 Function in Infants With Severe Respiratory Syncytial Virus Disease. J Infect Dis. 2018; 218(1): 75-83.
- Feng Z, Xu L, Xie Z. Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus. Front Cell Infect Microbiol. 2022; 12: 858629.
- Gerry S, Bonnici T, Birks J, et al. Early warning scores for detecting deterioration in adult hospital patients: systematic review and critical appraisal of methodology. BMJ. 2020; 369: m1501.
- Vali P, Underwood M, Lakshminrusimha S. Hemoglobin oxygen saturation targets in the neonatal intensive care unit: Is there a light at the end of the tunnel. Canadian journal of physiology and pharmacology. 2019; 97(3): 174-82.