SciBase Journals
SciBase Clinical and Medical Case Reports
ISSN 2995-5874
- Article Type: Case Report
- Volume 2, Issue 1
- Received: Jan 24, 2024
- Accepted: Feb 27, 2024
- Published Online: Mar 05, 2024
Granulomatous Mastitis in a Systemic Lupus Erythematosus Patient: Case Report
Lais Zanlorenzi²*; Nelzi Ferreira de Queiroz Junior¹; Thiago Alberto Fernandes Gomes dos Santos²; Barbara Stadler Kahlow²; Thelma Larocca Skare²
1Resident of Rheumatology, Service of Hospital, Mackenzie Evangelical University (HUEM), Brazil.
2Professor and Doctor of Rheumatology, Service of Hospital, Mackenzie Evangelical University (HUEM), Brazil.
*Corresponding Author: Laís Zanlorenzi
Professor and Doctor of Rheumatology, Service of Hospital, Mackenzie Evangelical University (HUEM), Brazil.
Email: laisznl@gmail.com
Abstract
Granulomatous Mastitis (GM) is an uncommon form of mastitis frequently associated with autoimmune diseases. This entity requires a differential diagnosis with breast tumor and infections and may be treated with glucocorticoids and methotrexate. We report a case of GM in a patient with systemic lupus erythematosus already using high doses of prednisone and immunosuppressants that responded to the use of doxycycline. Some pathophysiological, histological and treatment aspects are discussed.
Keywords: Granulomatous mastitis; Systemic lupus erythematosus; Glucocorticoid, Antibiotics.
Citation: Zanlorenzi L, de Queiroz NF Junior, dos Santos TAFG, Kahlow BS, Skare TL. Granulomatous Mastitis in a Systemic Lupus Erythematosus Patient: Case Report. SciBase Clin Med Case Rep. 2024; 2(1): 1016.
Introduction
Granulomatous Mastitis (GM) is considered a subtype of autoimmune mastitis that may present as a diagnostic challenge [1]. This entity is defined by its histological pattern that shows lobulocentric non-necrotizing granulomas, and epithelioid histiocytes and should be differentiated from infectious and neoplastic breast lesions [1]. Local inflammation induces the infiltration of immunocompetent cells to react with delayed-type hypersensitivity forming the granulomatous structure [2]. The concomitance of GM with other autoimmune disease seems to have prognosticvalue; according to Basim et al. [3]. The association with rheumatic diseases increases 4.5 times the risk of recurrence. Even in the cases which the GM appears isolated, the presence of arthralgias, erythema nodosum [4,6] and circulating autoantibodies such as antinuclear antibodies and rheumatoid factor are found, highlighting the underlying autoimmune nature [7]. Herein we describe a case of GM associated with Systemic Lupus Erythematosus (SLE) that offered treatment difficulties but finally answered to the use of thalidomide and doxycycline.
Case description
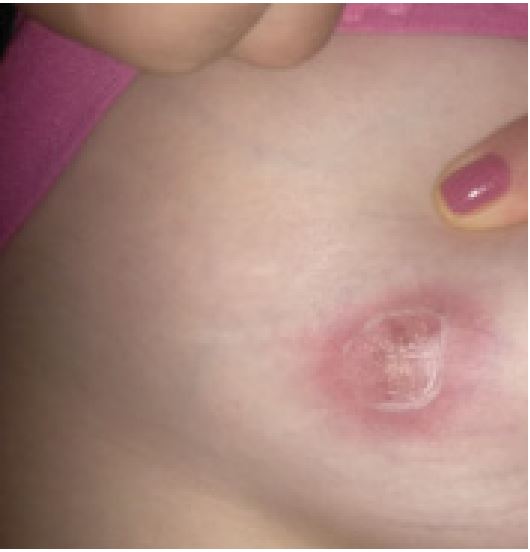
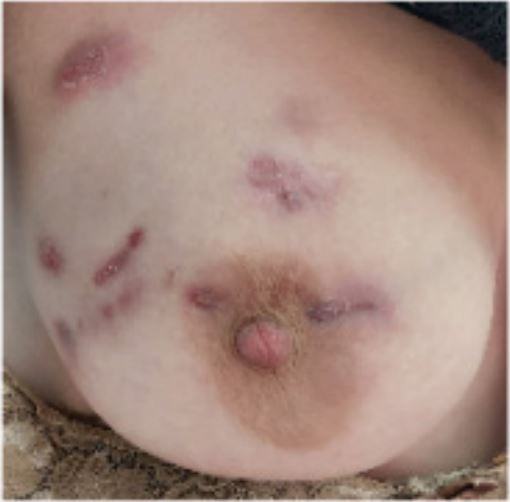
A 27 years old female was diagnosed having Systemic Lupus Erythematosus (SLE) based on presence of recurrent fever, photosensitive rash, non-cicatricial alopecia, vasculitis in the fingers, thrombocytopenia, positive antinuclear antibodies, anti-Sm and anti-dsDNA. She was treated with antimalarials and high dose glucocorticoid followed by mophetyl mycophenolate. She had no history of abortions and have had one child in 2018; she was using Intramuscular medroxyprogesterone since then. In November 2021, while on 60 mg of prednisone/ day, hydroxychloroquine 400 mg/day and 3 g/day of mycophenolate, she noticed a breast lump (Figures 1 and 2). Mammography and thorax MRI suggested a BIRADS 4 lesion, with no pulmonary findings. A core biopsy was performed and showed chronic granulomatous mastitis. The material was negative for acid fast bacilli and cultures also negatives. ANCA-c and p, IGRA (Interferon Gamma Release Assay) and ACE (Angiotensin I-Converting Enzyme) were negative. Prolactin level was normal (<10 ng/mL). As the patient was already using prednisone and mycophenolate, an attempted treatment with thalidomide was done. After 2 months of thalidomide use, some improvement was noted: the lesions seem softer, less painful and slightly smaller and paler. A biopsy review was done and the presence of some cystic spaces filled with neutrophils associated to the granulomas came into attention, reclassifying the lesion as cystic neutrophilic granulomatous mastitis, with Corynebacterium species possibly correlated. Long-term doxycycline was added with constant improvement until now.
Discussion
The present case has at least some peculiarities that need to be noted. The first one was the association with SLE. When a patient with SLE presents with mammary involvement the first diagnosis to be excluded is lupus panniculitis also known as lupus profundus [8]. This is a predominantly lobular panniculitis with a dense lymphoplasmacytic infiltration of lobules and hyaline fat necrosis that usually occurs in patients with a previous diagnosis of systemic or discoid lupus but that can also be the initial presentation of the disease [8]. Histological examination and the presence of overlying discoid skin lesions are some aspects used for differentiation between lupus panniculitis and GM [9,10]. A second interesting observation is that GM has been frequently described in patients with high levels of prolactin. [11].GM has been associated to prolactinomas [12], high levels of prolactin due to anti-psychotic drugs use [11] and in breastfeeding women [12].Nikolaev et al. [13].Described four cases of prolactin secreting tumor in patients with GM that had a complete resolution along with normalization of prolactin levels. Thus, abnormal levels of this hormone may be implicated in the physiopathology of GM. The prolactin has hormonal and a cytokine functional roles; it affects the immune system modulation preventing the negative selection of autoreactive B lymphocytes [14].Moreover, high prolactin levels have been described in relation to the pathogenesis and activity of several autoimmune disorders, among them, the SLE [14]. Nevertheless, this was not the present case in which prolactin levels were normal. A third interesting observation is about patient’s clinical response to treatment. Because its autoimmune background, glucocorticoids and methotrexate have been proposed as first line treatment [2,15]. The patient described presently was already using glucocorticoid and immunosuppressants for SLE treatment when she developed the GM. So, an attempted use of thalidomide was done. The rationality for using thalidomide was grounded in the presence of granulomas in which TNF-alpha is essential for the orderly enrolment of cells. Thalidomide has immunomodulatory properties; amid them, the suppression of TNF alpha effects [16]. In the described case, despite some improvement observed with thalidomide use, the response was considered very modest and doxycycline was added. More recently, the presence of Corynebacteria, especially Corynebacterium kroppenstedtii, has been implicated in the appearance of GM [2]. These microorganisms have been identified locally by culture and RNA gene sequencing and, according to some authors, they mark a subtype of GM called Cystic Neutrophilic Granulomatous Mastitis (CNGM) [2,17]. The CNGM has a peculiarity from histological point of view: it has vacuoles filled with neutrophils concomitant with the granulomas [2,18]. The role of Corynebacteria in this context is not clear. This microorganism is part of normal skin flora and their presence may be just the result of colonization [2]. Moreover, there is also the possibility that this bacteria offers an independent immunogenic factor or that is causing a secondary infection in a tissue with decreased local immune resistance, as an epiphenomenon [2]. Despite this uncertainty, the identification of Corinebacterium should lead to antibiotic use [2]. No microorganism has grown in the cultures of the presently described patient, nevertheless in the review of biopsy findings, some cystic spaces were detected suggesting CNGM and grounding the antibiotic use. Finally, the authors would like to bring to attention the association of GM with other autoimmune diseases. The knowledge of this association will help clinicians who cares for these patients to readily recognized and treat this disease.
Declarations
Ethical approval: This review was approved of Ethical Committee number: 65415922.6.0000.0103.
Consent to participate: Not applicable.
Consent for publication: Dear Editor, We wish to submit an original article entitled Granulomatous Mastitis in a Systemic Lupus Erythematosus Patient.
Availability of data and materials: Not applicable.
Competing interests: None.
Funding: Not applicable.
Authors’ contributions: Not applicable.
Acknowledgements: Not applicable.
References
- Goulabchand R, Hafidi A, Van de Perre P, Millet I, Maria ATJ, Morel J et al. Mastitis in autoimmune diseases: Review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med. 2020; 9(4): 958. doi: 10.3390/jcm9040958.
- Yuan QQ, Xiao SX, Farouk O, Du YT, Sheybani F, Tan QT, A et al. Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition). Mil Med Res. 2022; 9(1): 20.doi: 10.1186/s40779-022-00380-5.
- Basim P, Argun D, Argun F. Risk Factors for idiopathic granulomatous mastitis recurrence after patient-tailored treatment: Do we need an escalating treatment algorithm? Breast Care (Basel). 2022; 17(2): 172-179.doi: 10.1159/000517399.
- Sheybani F, Sarvghad M, Naderi, H, Gharib, M. Treatment for and clinical characteristics of granulomatous mastitis. Obstet. Gynecol. 2015; 125: 801-807. doi: 10.1097/AOG.0000000000000734.
- Salesi M, Karimifar M, Salimi F,Mahzouni P. A case of granulomatous mastitis with erythema nodosum and arthritis. Rheumatol. Int. 2011; 31: 1093-1095. doi: 10.1007/s00296-021-04820-8.
- Akın M, Karabacak H, Esendaglı G; Yavuz A, Gültekin S, Dikmen K et al. Coexistence of idiopathic granulomatous mastitis and erythema nodosum: Successful treatment with corticosteroids. Turk. J. Med. Sci. 2017; 47: 1590-1592. doi: 10.3906/sag-1611-100.
- Altintoprak F, Karakece E, Kivilcim T, Dikicier E, Cakmak G, Celebi F, Ciftci IH. Idiopathic granulomatous mastitis: An autoimmune disease? Sci. World J. 2013; 2013: 148727. doi: 10.1155/2013/148727.
- Rosa M, Mohammadi A. Lupus mastitis: a review. Ann Diagn Pathol. 2013; 17(2): 230-3. doi: 10.1016/j.anndiagpath.2012.09.003.
- Roongta R, Joshi S, Chattopadhyay A, Ghosh A. Lupus mastitis. Reumatol Clin (Engl Ed). 2022; 18(5): 312-313. doi: 10.1016/j.reumae.2021.05.006.
- AlQadri NG, AlNooh B, AlTewerki MM, Almotairi A, Alajlan S. Intravenous immunoglobulin in the management of lupus erythematosus panniculitis. Cureus. 2020; 12(1): e6790. doi: 10.7759/cureus.6790.
- Tian C, Wang H, Liu Z, Han X, Ning P. Characteristics and management of granulomatous lobular mastitis associated with antipsychotics-induced hyperprolactinemia. Breastfeed Med. 2022; 17(7): 599-604. doi: 10.1089/bfm.2021.0341.
- Bi J, Li Z, Lin X, Li F, Xu H, Yu X et al. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. International Journal of Infectious Diseases. 2021; 113: 243-250. doi: 10.1016/j.ijid.2021.10.019.
- Nikolaev A, Blake CN, Carlson DL. Association between hyperprolactinemia and granulomatous mastitis. Breast J. 2016; 22(2): 224- 31. doi: 10.1111/tbj.12552.
- Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and Autoimmunity. Front Immunol. 2018; 12: 9-73. doi: 10.3389/fimmu.2018.00073.
- Shojaee L, Rahmani N, Moradi S, Motamedi A, Godazandeh G. Idiopathic granulomatous mastitis: challenges of treatment in Iranian women. BMC Surg. 2021; 21(1): 206. doi: 10.1186/s12893-021-01210-6.
- Huang F, Wei JC, Breban M. Thalidomide in ankylosing spondylitis. Clin Exp Rheumatol. 2002; 20(6 Suppl 28): S158-61. PMID: 12463469.
- Bi J, Li Z, Lin X, Li F, Xu H, Yu X, et al. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int J Infect Dis. 2021; 113: 243-250. doi: 10.1016/j.ijid.2021.10.019.
- Wang L, Jorns JM. Cystic neutrophilic granulomatous mastitis: Corynebacterium species-associated infection with distinct histology. Clin Microbiol Infect. 2021; 27(2): 236-237. doi: 10.1016/j.cmi.2020.06.037.