SciBase Journals
SciBase Clinical and Medical Case Reports
ISSN 2995-5874
- Article Type: Research Article
- Volume 2, Issue 1
- Received: Jan 24, 2024
- Accepted: Feb 29, 2024
- Published Online: Mar 07, 2024
Chest CT Imaging Features of HIV/SARS-COV-2 Infected Patients from the Capital of Amazonas State
Paulo Nogueira*; Taynná Vernalha Rocha Almeida; Sildomar Queiroz e Silva; Daniella Paula Dias Coelho; André Patrício Ferreira de Almeida; Jackeline Andressa Barbiero; Maria Gabriela Teles de Moraes; Isadora Frota Hagge; Vanessa Vieira Pinheiro Corrêa; Sabrina Bianco; Sabrina Araújo de Melo; Alessandra Maria Paiva Gomes; Daniela Carla Menezes Genuíno; Carlos Henrique Michiles Frank; Paula Bonates Bessa; Andreza Karoline Barros Brito; Monique Freire dos Reis; Michele de Souza Bastos; Luiz Carlos de Lima Ferreira
State University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
*Corresponding Author: Paulo Nogueira
State University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
Email: pkochnogueira@gmail.com
Abstract
The impact of the COVID-19 pandemic caused by SARS-CoV-2 on global public health, emphasizing its severity and the efforts made to enhance diagnostic practices and clinical management. Chest Computed Tomography (CT) has been widely used to assess complications caused by the virus, particularly in patients coinfected with HIV. The study evaluated 29 chest CT scans, comparing HIV+ and HIV- patients. Among HIV+ patients, 66.7% reported regular antiretroviral therapy, and 43.8% had a CD4+ T cell count below 200 cells/mm3 . Coinfections were observed, including neurotoxoplasmosis and pulmonary tuberculosis. Clinical outcomes showed that 90% of patients were discharged, 3.5% were transferred, and 7% died, all of whom were HIV+ . CT findings revealed that HIV+ patients exhibited fewer ground-glass opacities, but a higher rate of consolidation. The severity score was lower in HIV+ patients, with the lower lobes of HIV- patients more affected. The text also discusses the variability in CT manifestations in HIV patients, highlighting the importance of considering previous conditions. The study suggests that HIVrelated immunosuppression may paradoxically protect against severe COVID-19 manifestation, but low CD4 counts (<200 cells/ µL) can predispose patients to more severe forms of the disease. However, the study acknowledges limitations, such as a small sample size and the need for follow-up CT examinations to understand the complete disease course.
Citation: Nogueira P, Almeida TVR, Silva SQE, Coelho DPD, Almeida APFD, et al. Chest CT Imaging Features of HIV/SARS-COV-2 Infected Patients from the Capital of Amazonas State. SciBase Clin Med Case Rep. 2024; 2(1): 1017.
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has become a major issue in the global public health sphere due to its high morbidity and mortality rates [1]. Since the initial global outbreak in December 2019 in the Chinese city of Wuhan, efforts have been made to gather evidence for improved diagnostic practices, clinical management, and healthcare attention. In the quest to assess complications caused by the novel coronavirus and to rule out other diagnoses, chest Computed Tomography (CT) has been widely employed [2]. However, there is a scarcity of data exploring the main CT findings in patients coinfected with the Human Immunodeficiency Virus (HIV) and SARS-CoV-2. It is believed that the presence or absence of lung lesions observed through chest CT in COVID-19 patients correlates with the severity of the infection and potential complications arising from pre-existing comorbidities [3]. The main CT findings reported in the literature to date, in descending order, include ground-glass opacities, ground-glass opacities associated with thickening of interlobular septa, characterizing a mosaic pattern, and groundglass opacities associated with consolidations [4]. In the same analysis, there are also numerous pulmonary complications related to opportunistic diseases associated with HIV, and their manifestations vary according to the patient’s degree of immunosuppression and the type of opportunistic diseases presente [5]. Successful diagnosis of these diseases depends not only on a thorough clinical history but also on the CT findings, which can be highly suggestive and often establish a probable diagnosis. Therefore, this study aims to assess HIV+ patients hospitalized for COVID-19 and analyze the presence or absence of patterns in these CT findings when compared to HIV- patients.
Materials and methods
Patients: Patients with confirmed SAR-CoV-2 infection through RT-PCR, rapid test, or serological testing, who were hospitalized at the Dr. Heitor Viera Dourado Tropical Medicine Foundation (FMT-HVD), were evaluated in two groups based on the presence or absence of HIV coinfection. This retrospective study received approval from the Human Research Ethics Committee of FMT-HVD (CAAE: 32785620.6.0000.0005, opinion number: 4.061.526).
Imaging acquisition: Within 24 hours of hospital admission, all individuals underwent a CT scan (Revolution CT – GE HealthCare) following a standard chest protocol, without the use of intravenous contrast. The patient was positioned in the dorsal decubitus with arms raised and instructed to hold their breath during acquisition to include the total lung volume. The scan time was approximately 2 seconds, and the slice thickness for reconstruction was 1.25 mm. The lung window configuration had a window level of -600 Hounsfield Units (HU) and a window width of +1500 HU.
Imaging evaluation: The CT images were assessed for the following characteristics: distribution of peripheral or peribronchovascular findings; presence of ground-glass opacities, mixed opacities, or consolidations only; presence of mosaic pattern or reversed halo sign; presence of air bronchogram, interlobular septal thickening, and cavities. These findings were classified according to the consensus recommendation of the North American Society for reporting chest CT findings related to COVID-19 [1].
All CT scans were evaluated by two radiologist physicians. The visual quantitative assessment of CT was based on the summary of acute inflammatory lung lesions involving each lobe, scored as none (0%), minimal (1-25%), mild (26-50%), moderate (51-75%), or severe (76-100%) involvement, with corresponding scores of 0, 1, 2, 3, or 4. The total severity score was calculated by summing the five scores for each lobe to quantify the extent of COVID-19 based on previously published studies [3,7-9]. For each patient, the “total severity score” of the CT was presented in the range of 0 to 20.
Statistical analysis: Data were tabulated in a database created by researchers using the Research Electronic Data Capture (RedCap) software, version 8.11.7. Descriptive analysis, with mean and standard deviation for normally distributed numeric variables and median for others, as well as the comparison between categorical variables using Pearson’s Chi-square test (significance level of 5%), were conducted using GraphPadPrism software (v7.0).
Results
Clinical data: A total of 29 chest CT scans were evaluated, with 16 from HIV+ patients (55%) and 13 from HIV- patients (45%). Among the HIV+ patient group, 10(66.7%) reported regular use of antiretroviral therapy, and 7(43.8%) had a CD4+ T cell count below 200 cells/mm3 . Complete values for CD4+ T cell count and viral load, as well as the therapeutic regimen, can be found in Supplementary Table 1. Tables 1 and 2 present the compiled data for both patient groups.
Table 1: General characteristics of the evaluated patients.
| Clinical data | HIV+ | HIV- |
|---|---|---|
| N=16 | N=13 | |
| Age* | 39(22-62) | 53(21-69) |
| Gender# | 9F(56.3%) 7M(43.8%) |
9F(69.2%) 4M(30.8%) |
| Days of symptom onset | 8.5(3-14) | 7.5(4-15) |
| Symptoms | ||
| Fever | 12(75%) | 10(76.9%) |
| Dry cough | 9(56.3%) | 10(76.9%) |
| Productive cough | 2(12.5%) | - |
| Dyspnea | 12(75%) | 12(92.3%) |
| Diarrhea | 7(43.8%) | 3(23.1%) |
| Vomiting | 3(18.8%) | 3(23.1%) |
| Dysgeusia | 2(12.5%) | - |
| Asthenia | 3(18.8%) | 3(23.1%) |
| Comorbidities | ||
| Arterial Hypertension | 1(6.3%) | 9(81.8%) |
| Diabetes | 3(18.8%) | 6(54.5%) |
| Obesity | 1(6.3%) | 2(18.2%) |
| Asthma | 1(6.3%) | - |
| Oxygenation support | ||
| Mechanical ventilation | - | 1(7.7%) |
| Low-flow nasal cannula | 5(31.3%) | 7(53.8%) |
Regarding coinfections, whether concurrent or not, 2 patients (20%) had neurotoxoplasmosis, 5 patients (50%) had pulmonary tuberculosis, 1 patient (10%) had Pneumocystis pneumonia, 2 patients (20%) had bacterial pneumonia, and 7 patients (70%) had oropharyngeal candidiasis. In the overall clinical outcome of the study, 26 patients (90%) were discharged, 1 (3.5%) was transferred to another institution, and 2 (7%) died, all of whom were HIV+ . The average length of hospital stay was 22 days for HIV+ and 10 days for HIV- .
Table 1: Laboratory data of the evaluated patients.
| Laboratory data | HIV+ | HIV- |
|---|---|---|
| Median (05 and 95 interquartile) | ||
| White blood cell (4-10×103/mm3) | 6(4.1-14.3) | 11(4.4-14) |
| Hemoglobin (12.5-15.5 g/dL) | 13(7.8-13.8) | 14(8.6-15.2) |
| Platelets (150-450×103/mm3) | 249(186-347) | 231(146-328) |
| Alkaline phosphatase (65-300 IU/L) | 340(193-617) | 222(140-302) |
Chest CT: Of the 29 evaluated patients, 17(58.6%) presented ground-glass opacities without consolidations, predominantly distributed peripherally. As expected, additional findings unrelated to COVID-19 were more frequently encountered in HIV+ patients. Detailed images are presented in Supplementary Table 2. A comprehensive comparative description of the CT evaluations can be found in Table 3.
Table 3: CT features in the twenty-nine patients with SARS- CoV-2.
| Parameters | HIV+ | HIV- |
|---|---|---|
| N=16 | N=13 | |
| Chest CT features | ||
| Ground-glass opacity only | 8(50%) | 9(69.2%) |
| Consolidation only | 6(37.5%) | 2(15.4%) |
| Mixed opacity | 2(12.5%) | 1(7.7%) |
| Crazy paving pattern | - | 2(15.4%) |
| Reversed halo sign | 1(6.25%) | - |
| Lesion distribution area | ||
| Peripheral distribution | 8(50%) | 8(61.6%) |
| Peribronchial distribution | 2(12.5%) | 2(15.4%) |
| Diffuse distribution | 6(37.5%) | 3(23.0%) |
| Additional findings | ||
| Air bronchogram | 2(12.5%) | 2(15.4%) |
| Centrilobular nodules | 3(18.8%) | - |
| Budding tree | 1(6.3%) | - |
| Cavitation | 1(6.3%) | - |
| Pleural effusion | 2(12.5%) | 1(7.7%) |
| Pulmonary emphysema | 1(6.3%) | - |
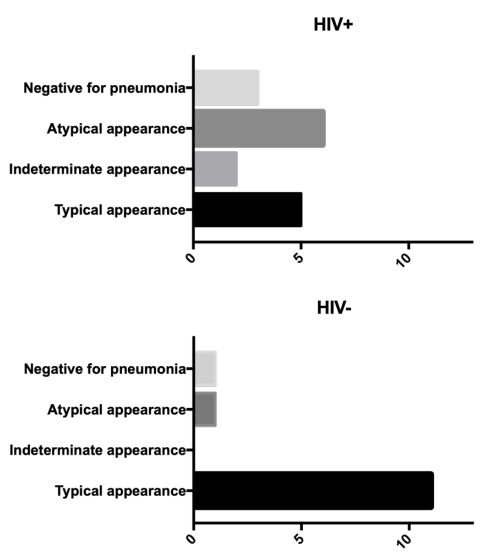
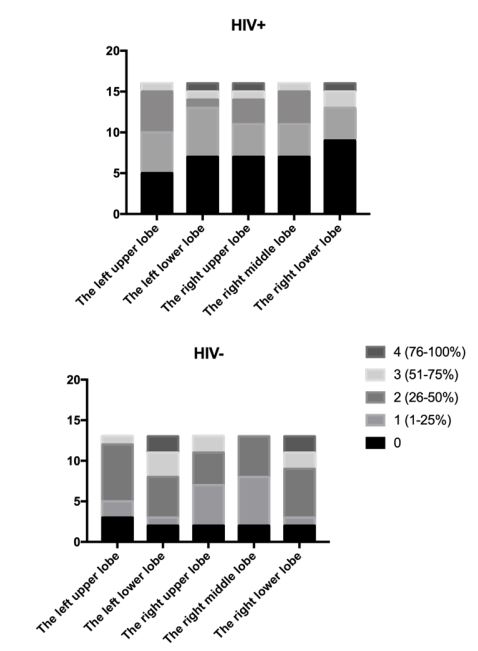
Regarding COVID-19 pneumonia, the classification result was statistically distinct (p=0.0351) between the evaluated groups and is presented in Figure 1. The involvement of COVID-19 lesions, distributed by lung lobe (Figure 2), mostly showed the lowest score (black) in HIV+ patients, with an average total severity score of 6.08, compared to 9.91 for HIV- . Regarding lobar involvement, there was a significant difference between groups in both lower lobes (right p=0.0439; left p=0.0380).
Discussion
Herein, we presented the case series of hospitalized HIV patients with COVID‐19 in a single hospital in the capital of Amazonas State. The clinical characteristics of COVID-19 did not appear to differ from those of the general population. However, atypical CT abnormalities representing COVID‐19 pneumonia were found between HIV+ and HIV- patients, differing of previuosly publications [6].
In our study, the presentation only with ground-glass opacities was less in HIV+ patients (50%) than in HIV- patients (69.2%), as well as less in relation to the meta-analysis carried out by Lee et al. [7] which demonstrated ground-glass opacities in HIV+ patients in a range from 68.8% (severe disease) to 72.2% (mild to moderate disease). However, the presentation with consolidation alone was much higher in HIV+ patients (37.5%) compared to HIV- (15.4%) in the present study, as well as in the review article by Awulachew et al. [8] in the general population (22%). HIV patients have a higher incidence of bacterial pneumonia, which is inversely proportional to the CD4 T cell count, when compared to the general population. Thus, bacterial pneumonia overlaid with COVID-19 is an important consideration.
Bilateral distribution of Ground Glass Opacities (GGO) with or without consolidation in posterior and peripheral lungs was the overriding indication of COVID-19 [9]. Nonetheless, with further analysis of accelerating cases, a diversity of interesting CT imaging features was found, including crazy paving pattern, airway changes, reversed halo sign etc., which can shed light on the possible mechanism of lung injury in COVID-19 [3,4,10,12].
Regarding illness involvement, our sample shows a statistical significance difference between HIV+ and HIV- group in both inferior lobes. The lower lobes of HIV- patients were more affected than that of HIV+ patients. In reviewing and analyzing the CT features of 62 cases of COVID-19 pneumonia, Zhou et al. [13] found that cases were most frequently seen to manifest as multiple lesions on the initial CT scan (83.9%); however, 16.1% of cases manifested as single lesion, and of those cases, 70.0% occurred within the inferior lobe of the proper lung. The authors further state that in comparison with early-phase disease, advanced-phase disease was related to a significantly increased frequency of GGO plus a reticular pattern, vacuolar sign, fibrotic streaks, air bronchogram, bronchus distortion, a subpleural line, a subpleural transparent line, and pleural effusion, but GGO was significantly decreased.
HIV+ patients had a lower severity score. As in the Awulachew et al. [8] study, HIV- patients showed greater involvement of the right lung. HIV+ patients had a greater impairment of the left lung. While in HIV-patients the left upper lobe was more affected, in HIV+ patients it was the most spared.
It is worth mentioning that there is a wide range of CT manifestations in HIV patient. The Pneumocystis carinii pneumonia, for example, has characteristics such as ground-glass opacity, scattered and ill-defined nodules, thickening of interstitial and interlobular septa, and irregular areas of air space consolidation. Pulmonary tuberculosis, on the other hand, presents classic findings such as infiltration in the upper lobe, endobronchial dissemination and excavations, in early stages, and diffuse infiltrates, multiple nodules, miliary lesions and mediastinal adenopathies, with border enhancement on CT with contrast in more severe phases. Fungal infections, in turn, have characteristics according to the type of infection, such as: candida, with nodules and lung cavities; cryptococcal pneumonia, with nodules, reticulonodular infiltration and mediastinal adenopathy; histoplasmosis, with small nodules; blastomycosis, with larger nodules, cavities and alveolar, interstitial and reticulonodular infiltrates associated or not with adenopathy. Bacterial infections can present findings such as: lobar and multilobe foci, cavitation, pulmonary necrosis and empyema and, in bacillary angiomatosis, endobronchial lesions, pulmonary nodules, mediastinal adenopathy, pleural disease and masses in the chest wall [5].
Gervasoni et al. [14] described 47 HIV-positive patients hospitalized by COVID-19 and concluded that HIV patients were not at greater risk of severe disease or death than HIV-negative patients. Motta et al. [15] compared 69 patients with SARS-CoV-2/ TB coinfections, although they might not show TB as a serious predictor of mortality in these patients. However, patients with previous lung disease like treated or untreated TB could affect the prognosis of patients with COVID-19, making greater vigilance necessary during outpatient follow-up [16].
The number of coinfection cases does not indicate that people living with HIV (PLWH) are at an elevated risk of COVID19 infection. A case study of five PLWH and COVID-19 showed that they were admitted at a younger age, but had positive clinical results overall [17]. Antiviral agents such as remdesivir, tenofovir, and lopinavir had antiviral activity against SARS-CoV-2 in previous studies, which led to the hypothesis that ART may provide defense against COVID19 in PLWH [18]. Despite many reports that certain antiviral agents boost COVID-19 symptoms [1], standard ART does not appear to protect PLWH from COVID19 at this time [19,20].
From our small sample, two HIV+ patients died. Given the immune system is significant contribution to the severity of COVID-19, especially the massive release of cytokines and chemokines, previous studies indicated that HIV-related immunosuppression could paradoxically protect against severe COVID-19 manifestation [21,22]. However, according to Kanwugu et al. [23], there is a clear connection between immune suppression (CD4 counts of less than 200 cells per µL) and increased disease severity (n=119; 2=7.772; P=.005) despite of the clinical outcome not significant (n=109; 2=1.191; P=.275). In a large population-based study using data from the OpenSAFELY platform in England [24], after accounting for demographic characteristics and lifestyle-related factors, PLWH are at more than double the risk of COVID-19 death as people without HIV. Any role HIV may play in raising the risk of infection or the development of severe disease is effectively ruled out by the inclusion of only hospitalized individuals who have already been infected with SARS-COV-2 and are likely to have severe disease at the time of inclusion [25]. In summary, contrary to earlier suggestions that coinfection patients may have mortality benefits from HIV‐related immunosuppression, low CD4 counts of less than 200 cells per µL can predispose them to more severe forms of the disease.
The following limitations of the present study should be mentioned: the number of cases is small; this is a case series of patients admitted to a tropical disease hospital with coinfections or prior illness; statistical tests and p values should be interpreted with caution because of the small sample size. Thus, studies that include follow-up CT examinations are needed to investigate the entire course of the disease.
References
- Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothorac Imaging [Internet]. 2020 Apr 1; 2(2): e200152. Available from: http://pubs.rsna.org/doi/10.1148/ryct.2020200152.
- de Smet K, de Smet D, Ryckaert T, Laridon E, Heremans B, Vandenbulcke R, et al. Diagnostic performance of chest CT for SARSCoV-2 infection in individuals with or without COVID-19 symptoms. Radiology. 2020; 298(1): 30-7.
- Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020.
- 4. Zu ZY, Jiang M Di, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020; 200490.
- Kuhlman JE. Imaging pulmonary disease in AIDS: State of the Art. Eur Radiol. 1999; 9(3): 395-408.
- Suwanwongse K, Shabarek N. Clinical features and outcome of HIV/SARS‐CoV‐2 coinfected patients in The Bronx, New York city. J Med Virol [Internet]. 2020 Nov ; 92(11): 2387-9. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jmv.26077.
- Lee KW, Yap SF, Ngeow YF, Lye MS. COVID-19 in People Living with HIV : A Systematic Review and Meta-Analysis. 2021.
- Awulachew E, Diriba K, Anja A, Getu E, Belayneh F. Computed Tomography ( CT ) Imaging Features of Patients with COVID-19 : Systematic Review and Meta-Analysis. 2020; 2020.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA [Internet]. 2020; 323(11): 1061. Available from: https://jamanetwork.com/journals/jama/fullarticle/2761044.
- Dai W-C, Zhang H, Yu J, Xu H-J, Chen H, Luo S-P, et al. CT Imaging and Differential Diagnosis of COVID-19. Can Assoc Radiol J [Internet]. 2020; 71(2): 195-200. Available from: http://journals.sagepub.com/doi/10.1177/0846537120913033.
- Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020; 1-6.
- Sahu KK, Lal A, Mishra AK. An update on CT chest findings in coronavirus disease-19 (COVID-19). Heart & lung : the journal of critical care. United States. 2020.
- Zhou S, Wang Y, Zhu T, Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. AJR Am J Roentgenol. 2020;1-8.
- Gervasoni C, Meraviglia P, Riva A, Giacomelli A, Oreni L, Minisci D, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis [Internet]. 2020. Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa579/5837155.
- Motta I, Centis R, D’Ambrosio L, García-García J-M, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology [Internet]. 2020; 26(4): 233-40. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2531043720301033.
- Gadelha Farias LAB, Moreira ALG, Corrêa EA, de Oliveira Lima CAL, Lopes IMP, de Holanda PEL, et al. Case report: Coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: Report of two cases. Am J Trop Med Hyg. 2020; 103(4): 1593-6.
- Jose L Blanco, Juan Ambrosioni, Felipe Garcia, Esteban Martínez, Alex Soriano, Josep Mallolas JMM. Correspondence COVID-19 in patients with HIV : clinical case series. 2020; 3018(20): 19-21.
- Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004; 59(3): 252-6.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020; 1-13.
- Härter G, Spinner CD, Roider J, Bickel M, Krznaric I, Grunwald S, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection [Internet]. 2020; 48(5): 681-6. Available from: https://doi.org/10.1007/s15010-020-01438-z.
- Shalev N, Scherer M, Lasota ED, Antoniou P, Yin MT, Zucker J, et al. Clinical Characteristics and Outcomes in People Living with Human Immunodeficiency Virus Hospitalized for Coronavirus Disease 2019. Clin Infect Dis. 2020; 71(16): 2294-7.
- Mascolo S, Romanelli A, Carleo MA, Esposito V. Could HIV infection alter the clinical course of SARS-CoV-2 infection? When less is better. J Med Virol [Internet]. 2020; 0-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32293709.
- Kanwugu ON, Adadi P. HIV/SARS-CoV-2 coinfection: A global perspective. J Med Virol. 2021; 93(2): 726-32.
- Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. HIV infection and COVID-19 death: a populationbased cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform Krishnan. 2020; 19-21.
- Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020; 11(1): 1-12.