SciBase Journals
SciBase Clinical and Medical Case Reports
ISSN 2995-5874
- Article Type: Case Report
- Volume 2, Issue 2
- Received: Apr 01, 2024
- Accepted: May 16, 2024
- Published Online: May 23, 2024
Use of Non-Invasive Elastographic and Microvascularization Biomarkers in the Diagnosis and Follow-Up of Children with Severe Bacterial Pneumonia
Sergi Huerta-Calpe1,2; Carmina Guitart1,2*; Bárbara Salas3; Francisco José Cambra1,2; Iolanda Jordan1,2; Monica Balaguer1,2
1Pediatric Intensive Care Unit, Sant Joan de Déu Hospital, Esplugues de Llobregat, Barcelona, Spain.
2Immune and Respiratory Dysfunction Research Group, Sant Joan de Déu Research Institute, Barcelona, Spain.
3Radiology and Diagnostic Imaging Unit, Sant Joan de Déu Hospital, Esplugues de Llobregat, Barcelona, Spain.
*Corresponding Author: Carmina Guitart
Pediatric Intensive Care Unit, Sant Joan de Déu Hospital, Esplugues de Llobregat, Barcelona, Spain.
Email: carmina.guitart@sjd.es
Abstract
Imaging tests such as Chest X-Ray (CXR) and Lung Ultrasound (LUS), and blood biomarkers like C-reactive protein and procalcitonin have demonstrated its role for Bacterial Pneumonia (BP) diagnosis. However, the implementation of novel biomarkers such as Lung Shear-Wave Elastography (LSWE) or the quantification of lung microvascularization ratio pattern by Superb Microvascular Imaging (SMI) has not yet been assessed. These biomarkers combined with the already described LUS findings, could be used to increase BP diagnosis accuracy, improving the sensitivity and specificity of its diagnosis. In this article, we describe the use of these non-invasive novel imaging biomarkers for the diagnosis approach in a pediatric patient with severe BP.
Keywords: Bacterial pneumonia; Lung ultrasound; Lung sono-elastography; Superb microvascular imaging.
Abbreviations: CXR: Chest X-Ray; LUS: Lung Ultrasound; BP: Bacterial Pneumonia; LSWE: Lung Shear-Wave Elastography; SMI: Superb Microvascular Imaging; ALRTI: Acute Lower Respiratory Tract Infections; PICU: Pediatric Intensive Care Unit; VP: Viral Pneumonia; HFOV: High-Frequency Oscillatory Ventilation; ECMO: Extracorporeal Membrane Oxygenation.
Citation: Huerta-Calpe S, Guitart C, Salas B, Cambra FJ, Jordan I, et al. Use of Non-Invasive Elastographic and Microvascularization Biomarkers in the Diagnosis and Follow-Up of Children with Severe Bacterial Pneumonia. SciBase Clin Med Case Rep. 2024; 2(2): 1022.
Introduction
Acute Lower Respiratory Tract Infections (ALRTI) are common in the pediatric population worldwide and often lead to Pediatric Intensive Care Units (PICU) admission. Among the different entities included in ALRTI, pneumonia is a very important cause of morbidity and mortality in children [1,2]. Generally, the most common symptoms of this condition are fever, cough, tachypnoea and respiratory distress, although in infants and young children the symptoms may be non-specific and may only present with weakness or feeding difficulties [3]. Pneumonia can be caused by bacterial, viral or fungal infections. Discerning the etiology of this condition is essential to decide the appropriate antibiotic treatment, when necessary, and thus to reduce antibiotic overuse and antibiotic resistance development. The final diagnosis is based on the combination of biochemical data, microbiological cultures, and imaging tests [4,5]. Those require invasive techniques due to the neediness of blood and respiratory samples extractions and provide ionizing radiation when using CXR, which is particularly problematic in pediatrics. Regarding imaging findings, BP typically presents as the presence of a lung consolidation, a finding that do not differentiate this condition from other different processes such as Viral Pneumonia (VP), atelectasis, pulmonary contusions or thromboembolism [6]. CXR might be considered the best diagnostic option in children and many institutions still rely on this ionizing technique for BP diagnosis. However, it shows low specificity regarding etiology diagnosis, it exposes patients to radiation and has limited diagnostic accuracy [7,8]. The available evidence suggests that LUS is a reliable, valuable, and alternative method for the diagnosis of pediatric pneumonia [8,9]. It can be performed at the patient’s bedside (point-of-care) and is radiation free [10,11]. Although LUS is known for its higher sensitivity and specificity, and its ability to provide evolutionary follow-up, it provides subjective and non-quantitative data. Therefore, it may not always distinguish between different lung consolidations (commonly found in BP) or interstitial patterns (commonly found in VP) [5,7,12-16].
Our research group is working to incorporate new non-invasive and quantitative diagnostic techniques for the diagnosis of BP, such as LSWE and SMI. Shear-wave elastography measures tissue elasticity. Assuming that soft tissues are more deformable than rigid tissues, lung elasticity values may differ depending on the different types of ALRTI [17,18]. This technique is commonly used in adults, particularly for studying organs such as the liver [19], breast [20], or thyroid [21]. However, its application over the lung conditions is still very limited. SMI is a recently developed ultrasound imaging technique that define the microvascular pattern from specific tissues based on the analysis of blood flow in very small diameter vessels, in order to translate it into a microvascularization rate value [22,23]. Like elasticity, non-invasive measurement of microvascularization rate may provide a quantitative measure useful to distinguish between different types of ALRTI. In this article we present case report of a patient with severe BP in which SWE and SMI were used as novel complementary diagnostic tools.
Case description
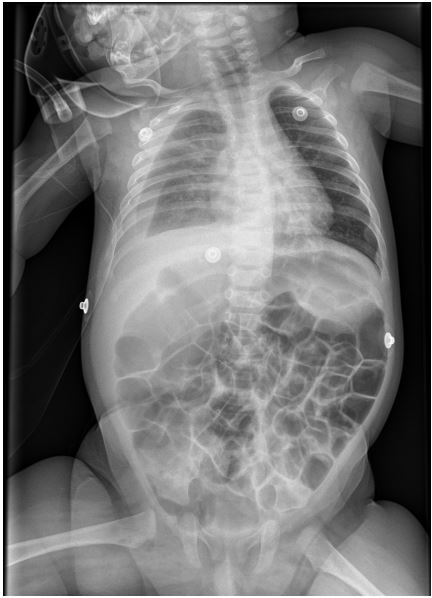
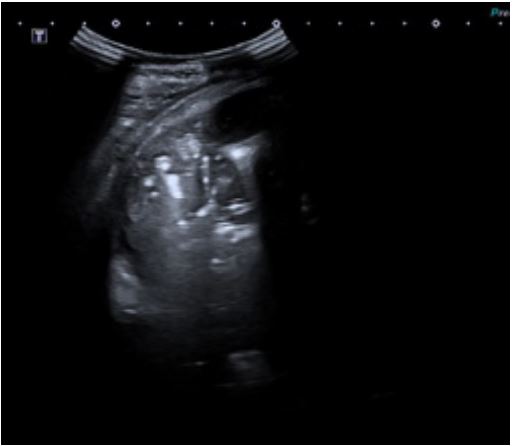
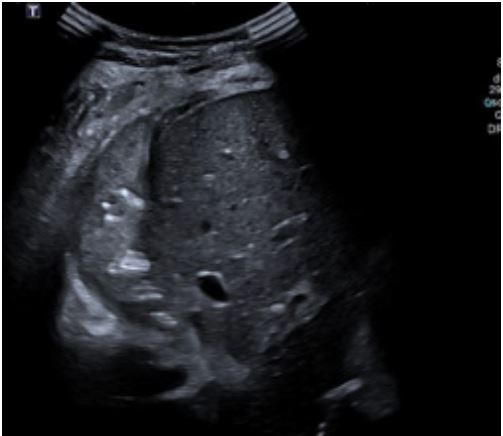
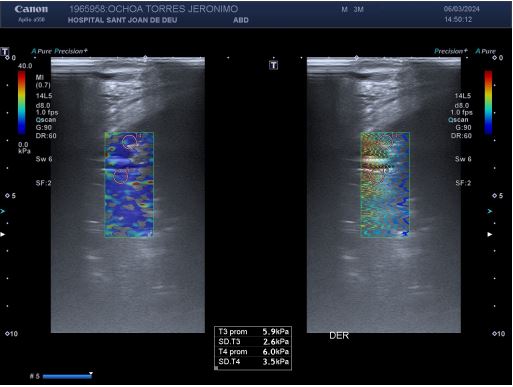
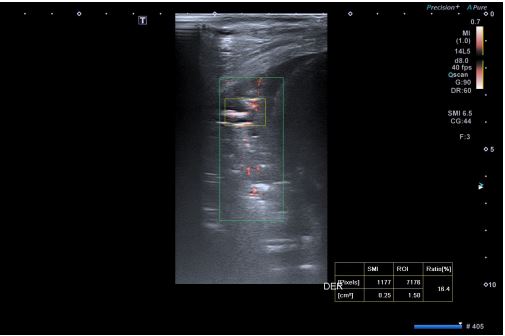
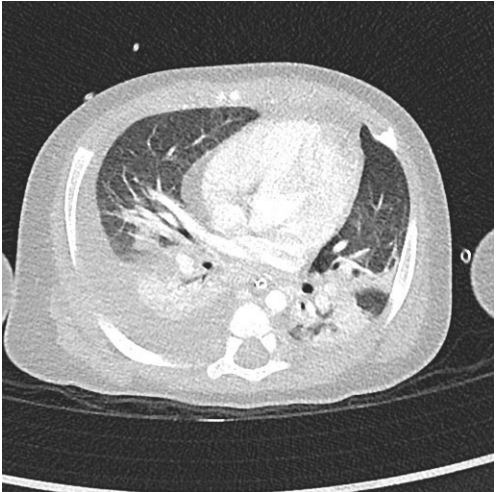
A four-year-old male, previously feet and well, presented with fever, weakness, vomiting and increased work of breathing. He was admitted to the PICU and required tracheal intubation due to progressive respiratory failure and associated hemodynamic instability. The blood test showed a hemoglobin level of 11 g/ dL, platelet count of 404,000/uL, and white blood cell count of 4,700/uL. Additionally, the C-reactive protein level was 241.3 mg/dL and the procalcitonin level was 194.04 ng/dL. The lactate value was 6 mmol/L and no coagulopathy was associated. A blood culture was obtained and a CXR (Figure 1) was performed at his admission, which revealed a right pleural effusion with no other apparent associated findings. The LUS (Figures 2a and 2b) showed severe hypoechogenic right basal consolidation with multiple internal air bronchograms, pleural membrane rupture and the associated pleural effusion. For further investigation of the lung affection, lung SWE (Figure 3) and SMI measurement (Figure 4) were performed, yielding an elastance of 6 KPa, and a microvascularization ratio of 16.4%, respectively. Both measurements were performed according to the approved lung imaging acquisition protocol of our institution [17]. Based on the analytical and ultrasound data, the measurement of an elastance value similar to that of normal liver tissue and a scarce microvascularization inside the consolidation, an infectious etiology was suggested. A drainage tube was inserted and a pleural sample was taken. Antibiotic treatment with cefotaxime was initiated after culture were taken. Additionally, a CT scan (Figure 5) was performed, revealing right necrotizing pneumonia with an associated parapneumonic effusion. Analysis of the samples revealed the presence of Staphylococcus aureus in both blood and pleural fluid. Due to the necrotizing characteristics of pneumonia and the patient’s severity, the antibiotic regimen was swapped to ceftaroline and clindamycin. Simultaneously, vasoactive support was commenced with a continuous adrenaline infusion pump. Twenty-four hours later, the patient presented respiratory deterioration due to right pneumothorax development. The patient exhibited persistent hypoxemia and hypercapnia despite conventional ventilation, so the decision was made to change the ventilatory approach to High-Frequency Oscillatory Ventilation (HFOV). However, there was no improvement in the patient’s condition. Due to the worsening clinical status, veno-venous Extracorporeal Membrane Oxygenation (ECMO) was initiated. Following the initiation of the therapy, the patient’s condition showed satisfactory progress and after 14 days of ECMO therapy it was determined that the patient no longer required extracorporeal circulation support. Subsequently, mechanical ventilation support could be withdrawn as the patient’s respiratory function improved.
Discussion
Respiratory infectious disorders are common in paediatrics and, especially BP, may be a frequent cause of life-threatening situations, as in the case presented. The diagnostic approach for BP is invasive, requiring analytical extractions and ionizing radiation from commonly used imaging tests. The routine use of LUS in most PICU has significantly improved non-invasive diagnosis. However, there are still situations where LUS alone cannot reliably determine the cause of certain lung consolidations. Additionally, LUS provides subjective data that may be influenced by inter-observer bias. Therefore, it is essential to incorporate new non-invasive diagnostic techniques that offer quantitative results, such as SWE or SMI [24-26]. If used sys . If used systematically, these biomarkers could introduce a new paradigm in the diagnosis and therapeutic approach of these patients. To achieve this, it is necessary to determine the normal values of these biomarkers in healthy lung tissue, with a view to eventually determining their values in tissues affected by BP. To this end, the first step could be to determine the pathological value of such biomarkers in confirmed BP cases by other tests such as CT scans or microbiological cultures. This publication provides an initial assessment of the pathological values of the biomarkers in lung tissue affected by BP. It is hypothesized that pneumonia-affected tissue is poorly distensible with limited aeration, resulting in high stiffness. Similarly, our hypothesis in relation to the measurement of the microvascularization ratio with SMI is that BP consolidations will have a lower microvascularization ratio given the hepatization of the tissue, although the presence of residual air within the tissue may make it difficult to measure this biomarker. The description of this case aims to introduce the presence of novel imaging diagnostic biomarkers that may aid in pneumonia diagnosis, optimize the treatment, and reduce radiation exposure in patients. Controlled clinical trials in the pediatric population are necessary to evaluate the potential usefulness of these techniques.
Declarations
Authors contributions: All authors have participated equally in all the stages of the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Consent for publication: The patient’s legal guardians have given their consent to the analysis of the patient’s case and the subsequent publication of the resulting data.
Funding: This research received no external funding.
Competing interests: The authors declare no conflict of interest.
Acknowledgments: Not applicable.
References
- Koh JWJC, Wong JJM, Sultana R, Wong PPC, Mok YH, et al. Risk Factors for Mortality in Children with Pneumonia Admitted to the Pediatric Intensive Care Unit: Risk Factors for Mortality in Pediatric Pneumonia. Pediatr Pulmonol. 2017; 52: 1076-1084. doi:10.1002/ppul.23702.
- Committing to Child Survival: A Promise Renewed : Progress Report Unicef: New York. 2015. ISBN 978-92-806-4815-7.
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, et al. On behalf of the British Thoracic Society Standards of Care Committee British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Children: Update. Thorax. 2011; 66: ii1-ii23. doi:10.1136/thoraxjnl-2011-200598.
- Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, et al. The Management of Community-Acquired Pneumonia in Infants and Children Older Than 3 Months of Age: Clinical Practice Guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical Infectious Diseases. 2011; 53: e25-e76, doi:10.1093/cid/cir531.
- Shah SN, Bachur RG, Simel DL, Neuman MI. Does This Child Have Pneumonia?: The Rational Clinical Examination Systematic Review. JAMA. 2017; 318: 462. doi:10.1001/jama.2017.9039.
- Berce V, Tomazin M, Gorenjak M, Berce T, Lovrenčič B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci Rep. 2019; 9: 17957. doi:10.1038/s41598-019-54499-y.
- Jones BP, Tay ET, Elikashvili I, Sanders JE, Paul AZ, et al. Feasibility and Safety of Substituting Lung Ultrasonography for Chest Radiography When Diagnosing Pneumonia in Children. Chest. 2016; 150: 131-138. doi:10.1016/j.chest.2016.02.643.
- Yan JH, Yu N, Wang YH, Gao YB, Pan L. Lung Ultrasound vs Chest Radiography in the Diagnosis of Children Pneumonia: Systematic Evidence. Medicine. 2020; 99: e23671. doi:10.1097/MD.0000000000023671.
- Ye X, Xiao H, Chen B, Zhang S. Accuracy of Lung Ultrasonography versus Chest Radiography for the Diagnosis of Adult Community-Acquired Pneumonia: Review of the Literature and MetaAnalysis. PLoS ONE. 2015; 10: e0130066. doi:10.1371/journal.pone.0130066.
- Orso D, Ban A, Guglielmo N. Lung Ultrasound in Diagnosing Pneumonia in Childhood: A Systematic Review and Meta-Analysis. J Ultrasound. 2018; 21: 183-195. doi:10.1007/s40477-018-0306-5.
- Rodriguez-Fanjul J, Benet N, Rodrigo Gonzalo De Lliria C, Porta R, Guinovart G, et al. Lung Ultrasound Protocol Decreases Radiation in Newborn Population without Side Effects: A Quality Improvement Project. Medicina Intensiva (English Edition). 2023; 47: 16-22, doi:10.1016/j.medine.2021.10.015.
- Buonsenso D, Musolino A, Ferro V, De Rose C, Morello R, et al. Role of Lung Ultrasound for the Etiological Diagnosis of Acute Lower Respiratory Tract Infection (ALRTI) in Children: A Prospective Study. J Ultrasound. 2022; 25: 185-197.
- Buonsenso D, Musolino A, Ferro V, De Rose C, Morello R, et al. Role of lung ultrasound for the etiological diagnosis of community- acquired pneumonia in children: a prospective study; Pediatrics. 2020.
- Conlon TW, Nishisaki A Singh, Y Bhombal, S De Luca, D Kessler, et al. Moving Beyond the Stethoscope: Diagnostic Point-of-Care Ultrasound in Pediatric Practice. Pediatrics. 2019; 144: e20191402. doi:10.1542/peds.2019-1402.
- Alejandre C, Balaguer M, Guitart C, Torrús I, Felipe A, et al. Procalcitonin‐guided Protocol Decreased the Antibiotic Use in Paediatric Patients with Severe Bronchiolitis. Acta Paediatr. 2020; 109: 1190-1195. doi:10.1111/apa.15148.
- Rodríguez-Fanjul J, Guitart C, Bobillo-Perez S, Balaguer M, Jordan I. Procalcitonin and Lung Ultrasound Algorithm to Diagnose Severe Pneumonia in Critical Paediatric Patients (PROLUSP Study). A Randomised Clinical Trial. Respir Res. 2020; 21: 255. doi:10.1186/s12931-020-01476-z.
- Huerta-Calpe S, Salas B, Inarejos Clemente EJ, Guitart C, Balaguer M, et al. Sono-Elastography: An Ultrasound Quantitative Non-Invasive Measurement to Guide Bacterial Pneumonia Diagnosis in Children. Children. 2023; 10: 1335. doi:10.3390/children10081335.
- Lim CK, Chung CL, Lin YT, Chang CH, Lai YC, et al. Transthoracic Ultrasound Elastography in Pulmonary Lesions and Diseases. Ultrasound in Medicine & Biology. 2017; 43: 145-152. doi:10.1016/j.ultrasmedbio.2016.08.028.
- Frulio N, Trillaud H. Ultrasound Elastography in Liver. Diagnostic and Interventional Imaging. 2013; 94: 515-534. doi:10.1016/j.diii.2013.02.005.
- Ricci P, Maggini E, Mancuso E, Lodise P, Cantisani V, et al. Clinical Application of Breast Elastography: State of the Art. European Journal of Radiology. 2014; 83: 429-437. doi:10.1016/j.ejrad.2013.05.007.
- Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, et al. US-Elastography in the Differential Diagnosis of Benign and Malignant Thyroid Nodules. 2008.
- Feng J, Lu J, Jin C, Chen Y, Chen S, et al. Diagnostic Value of Superb Microvascular Imaging in Differentiating Benign and Malignant Breast Tumors: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12: 2648. doi:10.3390/diagnostics12112648.
- Jin H, Wang C, Jin X. Superb Microvascular Imaging for Distinguishing Thyroid Nodules: A Meta-Analysis (PRISMA). Medicine. 2022; 101: e29505. doi:10.1097/MD.0000000000029505.
- Jiang Z, Huang Y, Shen H, Liu X. Clinical Applications of Superb Microvascular Imaging in the Liver, Breast, Thyroid, Skeletal Muscle, and Carotid Plaques. J of Ultrasound Medicine. 2019; 38: 2811-2820. doi:10.1002/jum.15008.
- Zhang X, Osborn T, Zhou B, Meixner D, Kinnick RR, et al. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans. Ultrason, Ferroelect, Freq. Contr. 2017; 64: 1298-1304. doi:10.1109/TUFFC.2017.2707981.
- Zhang X, Qiang B, Hubmayr RD, Urban MW, Kinnick R, et al. Noninvasive Ultrasound Image Guided Surface Wave Method for Measuring the Wave Speed and Estimating the Elasticity of Lungs: A Feasibility Study. Ultrasonics. 2011; 51: 289-295. doi:10.1016/j.ultras.2010.09.005.