SciBase Journals
SciBase Clinical and Medical Case Reports
ISSN 2995-5874
- Article Type: Case Report
- Volume 2, Issue 2
- Received: Apr 15, 2024
- Accepted: May 28, 2024
- Published Online: Jun 05, 2024
Endoscopic Closure of Duodenal Perforation using Intracavitary Endoscopic Negative Pressure Therapy
Milan Misovic1,2*; Braig C1 ; Weiner M3 ; Koyyala S4 ; Pielmayer S2,5; Muehldorfer S1,2
1Department for Gastroenterology, Klinikum Bayreuth GmbH, 95445 Bayreuth, Germany.
2Medizincampus Oberfranken, Friedrich Alexander University, Erlangen-Nürnberg, 95445 Bayreuth, Germany.
3Department of General Surgery, Klinikum Bayreuth GmbH, 95445 Bayreuth, Germany.
4Department of Radiology, Klinikum Bayreuth GmbH, 95445 Bayreuth, Germany.
5Department of Anaesthesiology, Klinikum Bayreuth GmbH, 95445 Bayreuth, Germany.
*Corresponding Author: Milan Misovic
Department for Gastroenterology, Klinikum Bayreuth GmbH, 95445 Bayreuth, Germany.
Email: misovic_m@yahoo.com
Abstract
Regardless of the cause of a duodenal perforation, operative treatment remains a fundamental therapeutic choice. The endoscopic closure of leaks is helpful only for fresh defects without an accompanying infection and is limited by the size of the defect. However, the operative treatment of duodenal perforations in the population of older and multimorbid patients is associated with a high morbidity, as well as mortality. Here, we describe a successful case of an endoscopic closure of a duodenal perforation in the bulbus using intracavitary endoscopic negative pressure therapy in a 68-year-old patient initially operated because of an acute cholecystitis with focal gallbladder perforation involving the duodenal wall. Over the course of time a secondary perforation of the duodenum was diagnosed, and a reoperation with a suturing of the duodenum did not lead to complete closure of the defect. Three weeks of intracavitary endoscopic negative pressure therapy led to the successful closure of the duodenal defect. This type of therapy can be an excellent alternative to surgery, for treating duodenal perforations in selected patients.
Keywords: Duodenal perforation; Endoscopic negative pressure therapy.
Citation: Misovic M, Braig C, Weiner M, Koyyala S, Pielmayer S, et al. Endoscopic Closure of Duodenal Perforation using Intracavitary Endoscopic Negative Pressure Therapy. SciBase Clin Med Case Rep. 2024; 2(2): 1023.
Introduction
Endoscopic Negative Pressure Therapy (ENPT) for postoperative suture insufficiency after surgical resections on the oesophagus or rectum, has been a standard therapy during the last decade [1,2]. Although this type of endoscopic therapy has been used for many years, and with a high percentage of success, it has not yet found its daily use in cases of other type of leaks in the gastrointestinal tract foremost due to technical and anatomical reasons, as well as risk of complications [3].
In the case of our patient, after the operative treatment of an acute perforated cholecystitis, the postoperative period was complicated by a duodenal perforation, which did not heal even after a surgical revision. Due to the patient’s multimorbidity, it was decided to perform ENPT using an open-pore film drain and so avoid a radical resection.
Case presentation
A 68-year-old male patient brought to the emergency department with a present h/o pain in the upper right abdomen associated with fever and vomiting. Symptoms were worsened in the last 12 hours. In addition to that the patient suffered from chronic atrial fibrillation, with a history of ischemic strokes. Due to the heart weakness and poor rhythm control, a pulmonary vein isolation was performed with an early recurrence of atrial fibrillation, and a pacemaker was implanted due to a sick sinus syndrome.
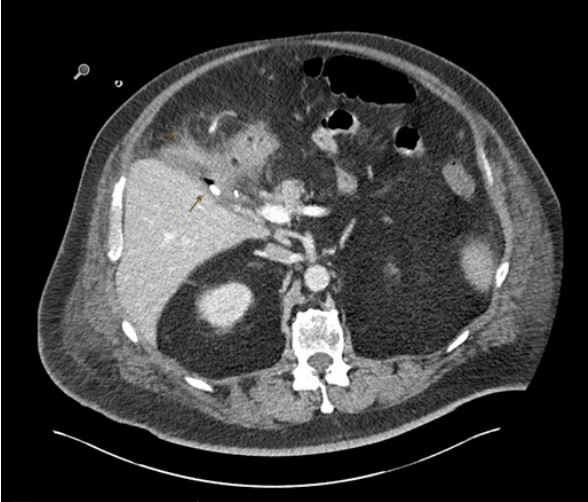
On examination patient was febrile, conscious, and semi oriented. Laboratory findings suggestive of an acute inflammation. Diagnostic CT-scan suggestive of an acute cholecystitis with perifocal abscess and a probable infiltration of adjacent duodenum.
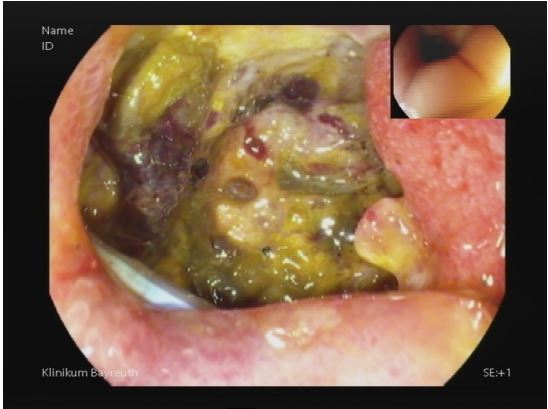
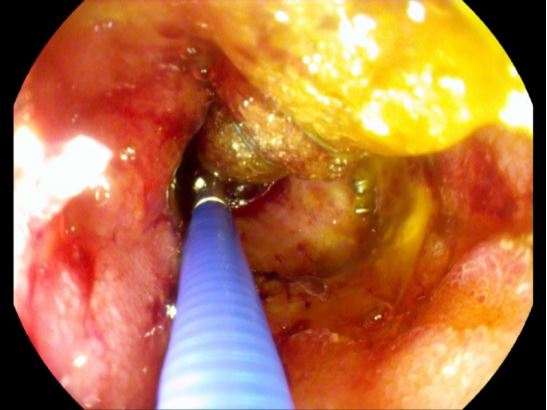
Hence, the patient was taken with prior consent for a primary laparoscopic cholecystectomy under general anaesthesia. Intraoperatively, a focally perforated gallbladder with a local abscess was found matching the findings of CT-Abdomen. The surgery was uneventful. Postoperatively due to sepsis patient kept under broad-spectrum antibiotic and was shifted extubated with a Robinson drain to intensive care unit. Despite of Antibiotic treatment patient showed continuous rise of inflammatory signs, additionally showed increased bile excretion in Robinson drainage. A diagnostic CT-abdomen revealed fluid and gas retention in the bed of gallbladder with signs of superinfection (Figure 1), furtherly diagnostic gastroscopy performed on secund postoperative day revealed a perforation in bulb of duodenum (Figure 2). The perforation was successfully closed due to laparoscopically reoperation on the same day. The patient was clinically stable under antibiotic therapy for the next three days and no bile contents were observed in the drainage. On forth postoperative day followed by revision operation, there was again an increase in signs of inflammation, and more significant amount of bile content was observed in the drainage. Further biochemical analysis confirmed a high concentration of bilirubin and lipase. A repeat gastroscopy performed on the same day revealed an ongoing leakage of previously known perforation in the bulb of duodenum due to a suture insufficiency (Figure 3).
Methods
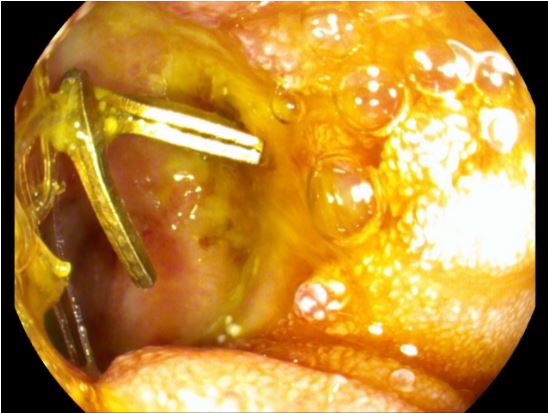
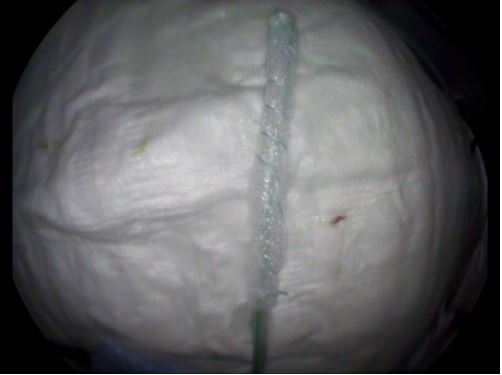
After the endoscopic removal of the surgical sutures and the clip, the opening of the perforation was intubated with a 9.9 mm gastroscope. A cavity 4-5 cm deep and 1-2 cm wide, whose walls were covered with necrotic material and fibrin, was seen. An endoscopic debridement of the cavern with a biopsy forceps was first performed (Figure 4a). Robinson’s drainage could be seen during debridement, and it was retreated by a few centimetres to the cavern’s edge. After that, a Trelumina tube was placed for the patient’s enteral feeding. A jejunal tube was placed deep into the small intestine, and the gastric part of the probe was placed all the way to the distal bulbous for it to have a drainage function. After that, the new drainage was made from the nasogastric tube by wrapping and sealing the distal 5 cm of the tube with foil, so that all the distal openings of the nasogastric tube were covered with foil (Figure 4b). This OpenPore Film Drain (OFD) was placed endoscopically in the perforating cavern (Figure 4c). The OFD tube passed through one nasal cavity and was connected to the vacuum pump, and the Trelumina tube passed through the other nasal cavity, through which the patient was fed enterally. The vacuum pump was set to a power of -125 mmHg. The patient was clinically stable with no signs of sepsis under the antibiotics.
Results
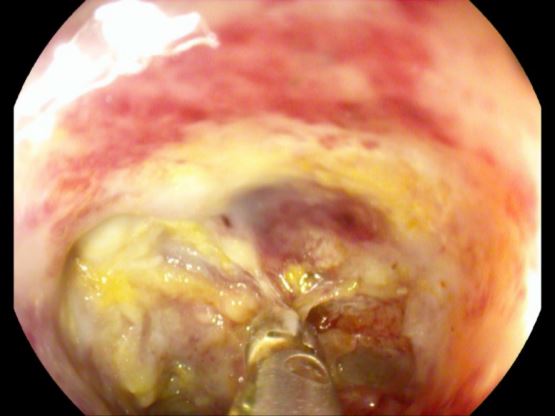
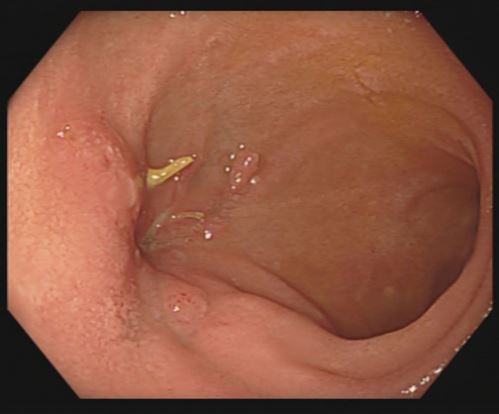
The OFD was changed twice a week for the next 2.5 weeks (total of 5 replacements), of which three replacements were performed under general anaesthesia when the drainage was placed inside the cavity, while the last two were performed under analgosedation when the OFD was placed in the lumen. Through the gastric supply of the Trelumina tube, there was no reflux of enteral nutrition and only a minor reflux of the bile content. Robinson´s drainage had less and less bile effusion every day until it stopped entirely after the third replacement of the OFD. After three weeks and after the sixth endoscopy, a decision was made to stop ENPT. Two control gastroscopies were performed the next month, and by the second control, a completely epithelialized lesion in the bulb of the duodenum could be seen (Figure 5).
Discussion
Regardless of the origin, duodenal perforations are complex to treat [4]. Duodenal injuries as a part of a laparoscopic cholecystectomy are very rare, with an incidence of 0,04% [5]. Nevertheless, this complication leads to a high rate of morbidity and mortality and needs a prompt closure to reduce it [4,6,7]. A primary surgical treatment does not always lead to the closure of the defect or it leads to new defects after the resection surgery is performed [8,9]. Endoscopic closure of perforations by clipping is limited by the size of the defect on the one hand and the timing of the endoscopy on the other, because only smaller, fresh, and non-infected perforations can be successfully treated this way [10].
Endoscopic Negative Pressure Therapy (ENPT) has been standardized and used since 2008 for the insufficient sutures, after the resection surgery of the oesophagus and rectum [1,2]. Over the last couple of years there is an increased number of publications about closures of duodenal perforations using the ENPT [11-14]. So far, two retrospective case studies of ENPT in duodenal perforations have been published. Intraluminal and intracavital applications of ENPT are both described, with the intraluminal application being significantly more frequent. In the above mentioned two studies, the success rate of this therapy is 80-100%. The main complication of this type of therapy is a significant bleeding due to injury to blood vessels, primarily in the case of intracaval application. This complication can be prevented by frequent drainage replacements [12,15]. The available literature shows that the application of ENPT in the duodenum is safe, but more extensive studies are needed for a more concrete conclusion. The disadvantage of this method is a relatively long period of treatment and the necessity of a constant availability of a trained and experienced endoscopic team. However, considering the above confirmed, positive effects of the treatment, when applied to the oesophagus and rectum, it should be chosen as a therapy option when dealing with duodenal perforations in selected patients.
Disclosure: The authors declare that they have no relevant or material financial interests that relate to the case report described in this paper.