SciBase Journals
SciBase Critical Care and Emergency Medicine
ISSN 2691-7785
- Article Type: Research Article
- Volume 2, Issue 1
- Received: Feb 05, 2024
- Accepted: Mar 20, 2024
- Published Online: Mar 27, 2024
Testing for Primary Hyperaldosteronism in Resistant Essential Hypertensive Patients
Mona Mansor1; Ahmed Rabie2; Fatma Abd-Elhafiz3; Randa Salam4; Marwa Fathy5; Abeer Awad Abdellatif6*
1Professor of Internal Medicine, Endocrinology & Diabetes, Kasr Al-Aini Hospitals, Faculty of Medicine, Cairo University, Cairo, Egypt.
2Associate Professor of Internal Medicine, Endocrinology & Diabetes, Kasr Al-Aini Hospitals, Faculty of Medicine, Cairo University, Cairo, Egypt.
3Lecturer of Clinical Pathology, National Cancer Institute, Cairo University, Cairo, Egypt.
4Professor of Internal Medicine, Endocrinology & Diabetes, Kasr Al-Aini Hospitals, Faculty of Medicine, Cairo University, Cairo, Egypt.
5Assistant Lecturer of Internal Medicine, Kasr Alainy Hospitals, Cairo University, Cairo, Egypt.
6Lecturer of Internal Medicine and Hepatogastroenterology, MD, Kasr Al-Aini Hospitals, Cairo University, Cairo, Egypt.
*Corresponding Author: Abeer Awad Abdellatif
Lecturer of Internal Medicine and Hepatogastroenterology, MD, Kasr Al-Aini Hospitals, Cairo University, Cairo Egypt. Internal Medicine department, Kasr Al-Aini Hospitals, Cairo University, Cairo Egypt.
Email: beero4a@yahoo.com
Abstract
Background: Hypertension is a common condition that affects many people all over the world. It could be associated with several complications especially in cases of resistant hypertension. Many clinical practice guidelines recommend screening for primary aldosteronism especially in persons with resistant hypertension owing to the worse prognosis when compared with blood pressure-matched essential hypertension.
Objective: The study aimed to screen for primary aldosteronism in high-risk hypertensive Egyptian patients, and to determine the challenges faced in the diagnosis.
Material and methods: 50 high-risk hypertensive patients were recruited from the Outpatient Endocrinology Clinic out of 200 hypertensive patients in the period from February 2019 and April 2021. Creatinine, Glycosylated hemoglobin (HBA1c), lipid profile, potassium level, sodium level, Plasma Aldosterone Concentration (PAC), Active Renin Concentration (ARC), and aldosterone/ Renin Ratio (ARR) were assessed in all patients.
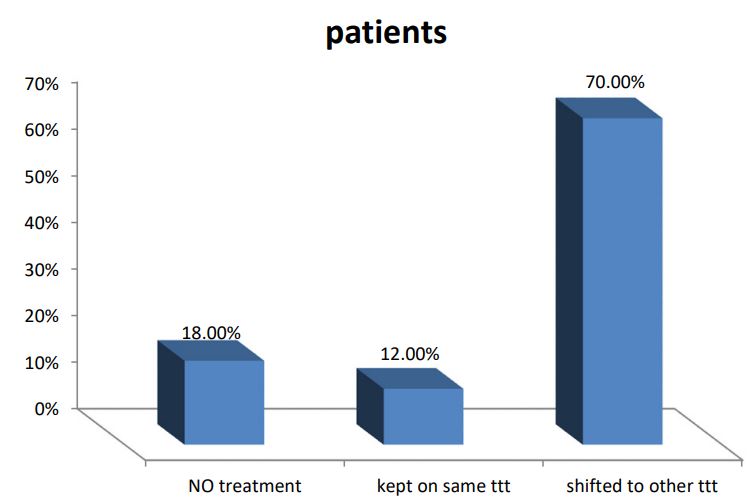
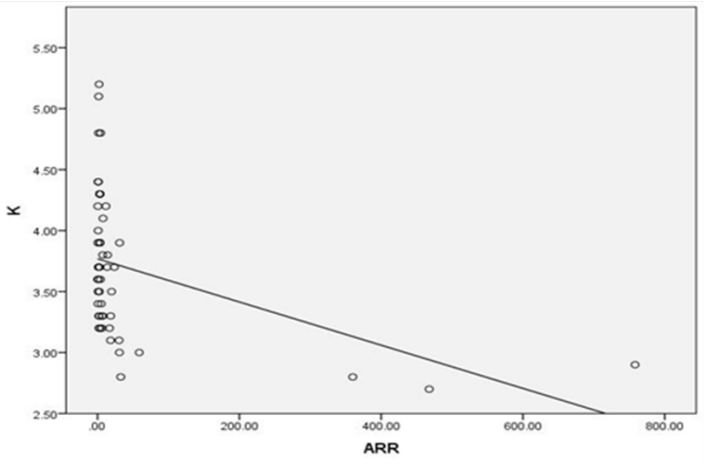
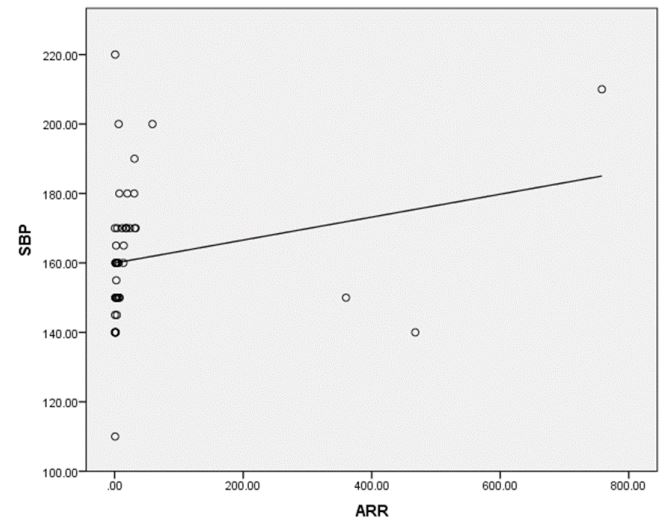
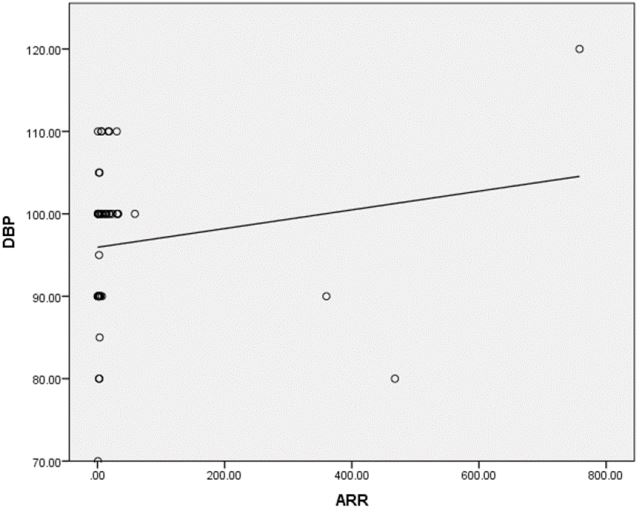
Results: A series of 50 hypertensive patients screened for PA (26 females and 24 males) with a mean age of 41.88±11.91 SD. We found that 41(82%) patients were receiving antihypertensive medications and 9(18%) patients didn’t receive treatment for hypertension previously. 9(18%) patients kept on no treatment, 6(12%) patients were kept on the same anti-hypertensive medications, and 35(70%) of them were shifted to other anti-hypertensive drugs. 4 patients out of total 50 patients had a positive ARR (>46), while 13(26%) patients out of total 50 hypertensive patients had low renin levels. There was a statistically significant relation between serum Aldosterone/ Renin Ratio (ARR) and serum Potassium (K) with P-value=0.001 (Figure 4), also a statistically significant relation between serum aldosterone/ renin ratio (ARR) and (systolic blood pressure, diastolic blood pressure) with P-value=0.001 was found.
Conclusion: We recommend routine screening for PA in high-risk hypertensive patients that could offer targeted treatment before adverse cardiovascular consequences develop.
Keywords: High-risk hypertensive patients; Primary aldosteronism; Plasma aldosterone concentration; Active renin concentration; Aldosterone/renin ratio.
Abbreviations: HBA1c: Glycosylated Hemoglobin; PAC: Plasma Aldosterone Concentration; ARC: Active Renin Concentration; ARR: Aldosterone/ Renin Ratio; PA: Primary Aldosteronism; PRA: Plasma Renin Activity; BAH: Bilateral Adrenal Hyperplasia; BMI: Body Mass Index; EH: Essential Hypertension; DBP: Diastolic Blood Pressure.
Citation: Mansor M, Rabie A, Abd-Elhafiz F, Salam R, Abdellatif AA, et al. Testing for Primary Hyperaldosteronism in Resistant Essential Hypertensive Patients. SciBase Crit Care Emerg Med. 2024; 2(1): 1004.
Background
Primary Aldosteronism (PA) has been reported to be 1-14% in the general hypertensive population and reaching up to 20% in populations with resistant hypertension [1]. In deed hypertensive patients with PA have higher rates of complications such as cardiovascular disease, kidney disease, and death when compared with patients with blood pressure-matched essential hypertension [2,3]. PA occurs as the result of a dysregulation of the mechanism controlling adrenal aldosterone production, leading to hypertension with low plasma renin and elevated aldosterone sometimes associated with hypokalemia. Among subtypes of PA, Aldosterone-Producing Adenoma (APA) and bilateral adrenal hyperplasia (also known as idiopathic hyperaldosteronism) together account for ≈95% of cases [4]. One of the key consequences of primary aldosteronism (PA) is the overproduction of aldosterone and the compensatory low renin levels. However, the term low-renin hypertension was initially proposed after studies dividing hypertensive patients into subgroups based on renin activity relative to urinary sodium excretion but is in many cases more or less a reflection of the sodium status [5]. Screening of suspicious cases of PA is usually started by measuring the plasma Aldosterone-to-Renin Ratio (ARR). Patients who have a positive ARR should undergo a confirmatory test with either saline infusion, fludrocortisone suppression, captopril challenge, or oral sodium loading to confirm or exclude the diagnosis [3]. The Plasma Aldosterone (PAC)-to Plasma Renin Activity (PRA) ratio as a screening test has been reported to be associated with a higher prevalence that is accounting for 10-32% of the patients with essential hypertension [6]. A subset of the population with Essential Hypertension (EH) has low renin, approximately 25 % [7]. In most of these Patients plasma aldosterone is normal, but the aldosterone–renin ratio becomes increased. Further support of low-renin hypertension and PA being in the spectrum of the same disease is a similar response to treatment with mineralocorticoid antagonists [8]. Not only the screening of PA for diagnosis but it also offers targeted treatment, either in the form of unilateral adrenalectomy for APA, or mineralocorticoid receptor antagonists for APA or more usually, for Bilateral Adrenal Hyperplasia (BAH) [9]. Cost should not be a barrier for screening adherence of primary aldosteronism in facing of cost of cardiovascular risk averted [10].
Aim of the work: The study aimed to screen for primary aldosteronism in high-risk hypertensive Egyptian patients and to determine the challenges faced in the diagnosis.
Material and methods
This study was enrolled 50 high-risk hypertensive patients recruited from the Outpatient Endocrinology Clinic out of 200 hypertensive patients in the period from February 2019 and April 2021. The study was approved by our institution’s Research Ethics Committee and informed written consent was obtained from all participated patients. All method details were conforming to Endocrine Society Clinical Practice Guideline, 2008.
High-risk patients were selected for PA screening depending on the presence of any of the following conditions:-
- Patients with Joint National Commission stage 2 (>160– 179/100-109 mmHg) and stage 3 (>180/110 mmHg).
- Drug-resistant hypertension (that is defined as suboptimally controlled hypertension on a three-drug program that includes an adrenergic inhibitor, vasodilator, and diuretic).
- Hypertension and spontaneous or diuretic-induced hypokalemia.
- Hypertension and a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 y).
- All hypertensive first-degree relatives of patients with PA.
The following patients were excluded:
- Patients aged >65 years, as renin can be lowered more than aldosterone by age alone, leading to a raised ARR.
- Renal failure patients as it can lead to false-positive ARR.
All the participants were subjected to detailed history including smoking, presence of diabetes mellitus, family history of early-onset hypertension, and/or cerebrovascular accident at a young age (<40 y). Drugs received by each patient were also recorded. Clinical examination was performed with special emphasis on assessment of Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and Body Mass Index (BMI). BMI was calculated according to patient’s height and weight {BMI = Weight / (height) 2} expressed in units of kg/m2.
All the participants were subjected to the following investigations: Creatinine, Glycosylated hemoglobin (HBA1c), lipid profile (total cholesterol, HDL, LDL, VLDL, and triglyceride), potassium level, and sodium level. According to the potassium level, potassium supplements were prescribed and the patients were advised to liberalize rather than restrict salt intake. Patients on antihypertensive medications were switched on one or more of the following drugs: Verapamil, Hydralazine, Prazosin, Doxazosin mesylate, Terazosin hydrochloride Spironolactone, eplerenone, amiloride, triamterene, Potassium wasting diuretics, and products derived from licorice root were stopped for 4 weeks. NSAIDs and other anti-hypertensive medications were stopped for 2 weeks. Plasma Aldosterone Concentration (PAC), Active Renin Concentration (ARC), and Aldosterone/ Renin Ratio (ARR) were assessed in all patients.
On the next visit, ARR was calculated at the outpatient clinic by measuring both PAC and ARC. An enzyme immunoassay for the quantitative measurement of aldosterone and renin levels in serum was used, taking the following precautions:
• Blood collection was done 8-10 am after the patient had been up (sitting, standing, or walking) for at least 2 h and seated for 5-15 min.
• Blood collection was done carefully, avoiding stasis and hemolysis.
• Samples were kept at room temperature during delivery to laboratory and before centrifugation with rapid freezing of plasma component pending assay.
Calculation of ARR: Plasma Aldosterone to Renin Ratio (ARR) is the ratio of Plasma Aldosterone Concentration (PAC, expressed in ng/L) to Plasma Renin Activity (PRC, expressed in ng/L/). ARR = (PAC (ng/L))/ (PRC (ng/L))
Serum Aldosterone/Active renin concentration ratio 20-40 indicates probable primary aldosteronism [11].
ARR is the most reliable means to screen PA, but a unique cutoff is difficult to define because of the wide variability of laboratory assays and population-specific characteristics.
Statistical analysis: Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 25. Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Mann-Whitney test [12]. Correlations between quantitative variables were done using the Spearman correlation coefficient [13]. P-values less than 0.05 were considered statistically significant.
Results
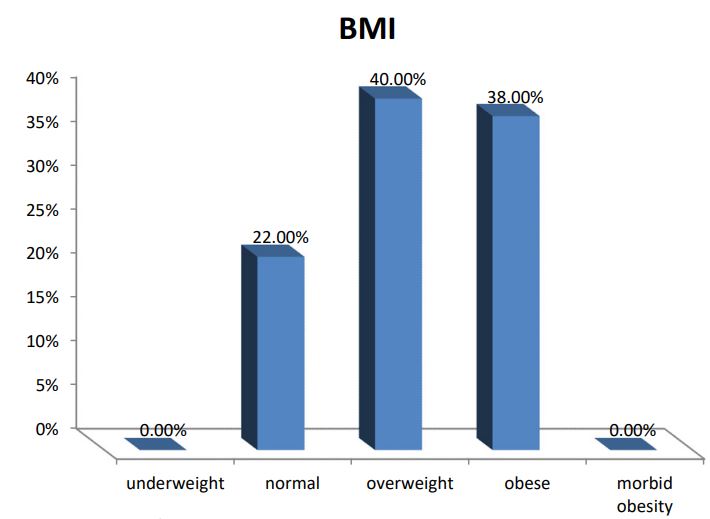
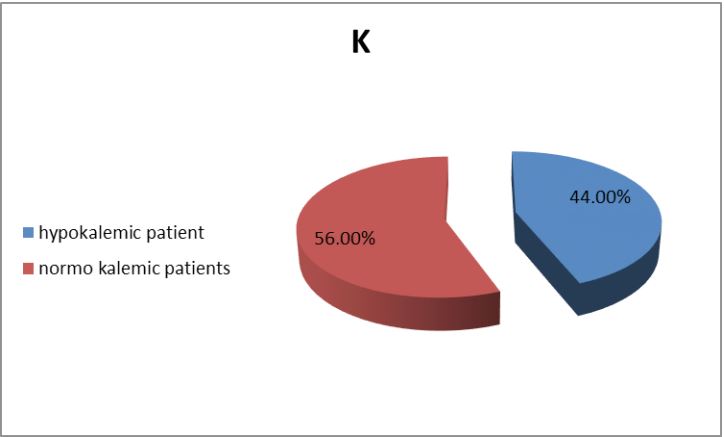
A series of 50 hypertensive patients screened for PA (26 females and 24 males) with a mean age of 41.88±11.91 SD. From the total 200 patients, 70 patients were excluded due to renal impairment, 45 patients were excluded due to their age which was >65, and the other 35 patients refused to shift medications to other forms. In our study, we found that 18(36%) of the patients had a positive family history of early-onset hypertension or cerebrovascular accident at a young age (<40 y), and 32(64%) patients had a negative history. We also reported that 41(82%) patients were receiving antihypertensive medications and 9(18%) patients didn’t receive treatment for hypertension previously. 9(18%) patients kept on no treatment, 6(12%) patients were kept on their same anti-hypertensive medications, and 35(70%) of them were shifted to other anti-hypertensive drugs (e.g. verapamil and α blockers), Figure 1. The characteristic of the studied patients Were demonstrated in Table 1. 40% of our patients were diabetic and 30(60)% non-diabetic. The Body Mass Index (BMI) of the patients were ranged between 19 and 40 kg/m2 with mean ± SD = 29.02±4.55. There were 11(22%) patients with normal BMI, 20(40%) overweight patients, and 19(38%) obese (Figure 2). Serum potassium levels (K) in our studied patients were ranged between 2.7 and 5.2 mEq/L with mean ± SD = 3.7±0.59 mEq/L. 22 patients were hypokalemic and 28 patients were normokalemic (Figure 3). Serum aldosterone (PAC) of our patients were ranged between 5.3 and 915.5 ng/L with mean ± SD = 118.02±173.37 ng/L, meanwhile direct renin (ARC) Were ranged between 0.11 and 276.1 ng/L with mean ± SD = 47.79±74.54 ng/L, and serum Aldosterone/direct renin ratio (ARR) were ranged between 0.1 and 758.1 with mean ± SD = 40.04±131.95. In our study; 9 patients were recently diagnosed on no treatment, 6 patients were kept on their same anti-hypertensive medications, and 35 of them were shifted to other antihypertensive drugs (e.g. verapamil and α blockers), 4 patients out of total 50 patients had a positive ARR (>46), while 13 (26%) patients out of total 50 hypertensive patients had low renin levels. There was a statistically significant relation between serum Aldosterone/ Renin Ratio (ARR) and serum Potassium (K) with P-value=0.001 (Figure 4), also a statistically significant relation between serum Aldosterone/ Renin Ratio (ARR) and (SBP, Diastolic blood pressure) with P-value=0.001 was found (Figures 5 and 6).
Table 1: The characteristic of the studied patients.
| Variable | Mean |
|---|---|
| Age | 41.88±11.91 |
| Family history | 18 (36%) |
| BMI | 29.02±4.55 |
| Waist circumference (cm) | 100.3±1.55 |
| Systolic BP (mmHg) | 161.3±19.66 |
| Diastolic BP (mmHg) | 96.4±9.42 |
| Serum sodium (mEq/L) | 137.06±3.66 |
| Serum potassium (mEq/L) | 3.7±0.59 |
| Serum creatinine (mg/dl) | 0.88±0.21 |
| Triglycerides (mg/dl) | 165.36±80.77 |
| Cholesterol (mg/dl) | 136.18±42.38 |
| HA1C % | 6.46±0.89 |
| Serum aldosterone (ng/L) | 118.02±173.37 |
| Active renin concentration (ng/L/) | 47.79±74.54 |
| Serum Aldosterone/active concentration | 40.04±131.95 |
Table 2: Characteristic of suspected PA patients.
| Patient | Sex | Age | Treatment | BP | BMI | A1C | Potassium | ARC | PAC | ARR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | female | 39 | none | 210/120 | 36 | 6.4 | 2.9 | 0.19 | 68.5 | 360 |
| 2 | female | 63 | C, D, BB | 170/110 | 32 | 8.5 | 3.2 | 0.11 | 83.4 | 758.1 |
| 3 | female | 64 | A | 200/100 | 30 | 6.4 | 3 | 2.1 | 123.5 | 58.8 |
| 4 | male | 54 | none | 170/100 | 20 | 8.7 | 2.8 | 0.19 | 88.9 | 467.8 |
Table 3: Correlation between ARR and studied parameters.
| ARR | Correlation coefficient | P value |
|---|---|---|
| Serum Potassium | -.516- | 0.001* |
| Systolic blood pressure | 0.533 | 0.001* |
| Diastolic blood pressure | 0.357 | 0.011* |
| LDL | .035 | .811 |
| A1C | .199 | .166 |
Discussion
PA has been reported as a relatively common endocrine cause of secondary hypertension, with a prevalence of 1-2% among unselected individuals with hypertension, although some groups have suggested this may be as high as 10-14% among selected specialist clinic populations with resistant hypertension [3,14]. however it is still under-diagnosed in a realworld setting [3]. Because PA may be either asymptomatic or presented with nonspecific symptoms particularly in its early stages and the deleterious effects of aldosterone overproduction are often reversible, thus, screening of patients with resistant hypertension at increased risk of primary aldosteronism is beneficial [15]. The diagnosis of PA is a step-wise approach that starts by screening, confirmation and subtype classification [3]. In our study, out of 200 hypertensive patients, only 50 patients were included (26 females and 24 males) with a mean age of 41.88±11.91 SD and 4 of them (8%) had a positive ARR (>46). This was in agreement with Gonzaga CC et al. [14] who stated that PA is the most common form of secondary hypertension, with an estimated prevalence between 6% and 12% of hypertensives. Also, Kline GA et al. [16] concluded that PA can be considered one of the leading causes of secondary hypertension, accounting for 5-10% of all hypertensive patients and more strikingly 20% of those with resistant hypertension. Although the guidelines do not specify which ARR cut‐off value should be used to define a positive result cutoff, commonly placed between 20 and 40 (in [ng/dL]/ [ng/mL per hour] for Plasma Aldosterone Concentration [PAC] and Plasma Renin Activity [PRA] [17]. Detection of increasing ARR values are associated with an exponential increase of the likelihood of an APA [18]. Such liberal cutoffs maximize sensitivity but generate many false-positive results, hence the Endocrine Society and the Japanese guidelines concur in supporting the use of confirmatory tests. Lack in using a uniform testing protocol or cut-off values across centers impeding an accurate, and consistent, diagnosis of PA in all affected patients [17]. The systematic use of confirmatory tests in clinical practice increases times, complexity, and costs of the diagnostic workup, thus contributing to the underdiagnosis of PA high ARR values could be associated with a high likelihood of APA [17]. Hypokalemia (defined as serum K+ <3.6 mEq/l) was prevalent in 22 patients out of 50(44%) in the overall study population. Patients with a positive ARR (8%) had K levels ranging from 2.7 to 3 mEq/l. In our study, there was a highly statistically significant relation between potassium level and plasma Aldosterone to Renin Ratio (ARR) (P<0.001). Among all the patients of the study group, the lower the potassium level, the higher the ARR. A retrospective study that was conducted on patients with PA who were diagnosed based on a combination of diagnostic tests and surgical outcomes, concluded that ARR had greater sensitivity and specificity than either aldosterone levels or PRA alone [19]. On the other hand, Harvey AM, et al. reported that hypokalemia should not be considered a diagnostic feature of PA [20]. In some studies, only a minority of patients with PA (9-37%) had hypokalemia. thus normokalemic hypertension constitutes the most common presentation of the disease; hypokalemia is probably present in only the more severe cases. In our study, we found that low renin hypertension was prevalent in 13 patients out of 50(26%) in the overall study population. Similarly, this goes with Adlin et al. who found that a subset of the population with Essential Hypertension (EH) has low renin, approximately 25%. In most of these Patients plasma aldosterone is normal, but the aldosterone–renin ratio becomes increased [7]. Interestedly, a study conducted from five international referral centers following the use of ARR as a routine screening test reported a 5- to 15-fold increase in the identification of PA 21. further support of low-renin hypertension and PA being in the spectrum of the same disease is a similar response to treatment with mineralocorticoid receptor antagonists [8]. Also, low-renin essential hypertension is typically salt and volume sensitive indicating a good response to diuretics and dietary modifications. In addition, there was a statistically significant relation between ARR and Systolic Blood Pressure (SBP) (P<0.001), as well as, between ARR and Diastolic Blood Pressure (DBP) (P<0.011). A study conducted by Calhoun et al. providing data on subjects with resistant hypertension found that the prevalence of PA is even higher, reaching 20% of studied subjects. Thus, the spectrum of PA in EH seems to be continuous from low frequencies in mild hypertensives, similar to those found in normotensive subjects, to very high frequencies in severe or resistant hypertension [6]. In our study HBA1C was of mean ± SD = 6.46±0.89% (prediabetic) despite the high prevalence of resistant hypertension among diabetic patients, the prevalence of primary aldosteronism is not known because screening for primary aldosteronism is seldom performed. Observed a prevalence of 14% of primary aldosteronism in diabetic subjects with poorly controlled hypertension while taking >3 antihypertensive agents. A growing number of studies have linked PA with metabolic syndrome, diabetes, and obstructive sleep apnea that may be partly responsible for the higher rate of cardio and cerebrovascular accidents in PA patients [22]. The strengths in our study were the use of Endocrine Society guidelines, 2008 to diagnose PA and screen all patients with ARR which is considered the most reliable means to screen for PA, also, Active Renin Concentration (ARC) was used as a measurement of ARR during PA screening instead of Plasma Renin Activity (PRA). Furthermore, to increase the test’s accuracy, we used a washout period for drugs that could affect the renin-angiotensin system and secondarily modify the ARR, which might yield false-negative or false-positive results. A false-negative result was communicated by Stowasser M et al. [23] with the use of angiotensin II receptor blockers and other antihypertensive drugs that were suspended 15 days before the study. We have several limitations in our study first was the smaller number of patients included in the study, the second was that the possibility of a false-positive result was not excluded with any of the confirmatory tests that should be performed in patients with a high PAC/PRC ratio and third was that we did not demonstrate the cardiovascular morbidity and mortality amongst patients with PA compared with blood pressure-matched essential hypertension.
Conclusion
Screening for primary aldosteronism in individuals who both fulfill the criteria for diagnosis of resistant hypertension, and have unprovoked hypokalaemia of PA places a high value on avoiding forgoing the opportunity for surgical or medical cure thus ameliorating high cardiovascular morbidity and should be given the priority for PA screening. ARR also considers a simple reliable screening test for PA.
Recommendations: There is a strong need for screening and further workup to diagnose primary aldosteronism especially in high-risk hypertensive patients; also we need to highlight an opportunity to implement proper screening tests and to alert the primary care providers and selected specialists to the importance of applying screening for PA for those high-risk patients owing to consideration of a treatable risk factor for cardiovascular disease. Also, raising the awareness of PA as a treatable cause of hypertension is a must especially amongst clinicians who manage hypertension. Also, we recommend further confirmatory tests in patients with high ARR that may offer the possibility of surgical cure or targeted pharmacotherapy with mineralocorticoid receptor antagonists. An alternative strategy with patients displaying high ARR and relatively mild hypertensive disease could be strict adherence to a low-sodium diet and low-dose MRA therapy. Further studies conducted on a much bigger number of patients are also needed.
Declarations
Ethics approval and consent to participate: All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. An informed consent was obtained from the legal representative of the participants included in the study. The ethical commitue approval number and date is not available.
Consent for publication: Oral and written informed consents were obtained from the patients or from their eligible relatives.
Availability of data and material: Not applicable.
Competing interests: The authors declare no potential competing interests.
Funding: Authors received no funding for this study.
Authors’ contributions: MM analyzed and interpreted the patients’ data and revised the final manuscript.
FA participated in interpretation of the data of the patients.
AR analyzed and interpreted the patients’ data.
MF, and RA collected the data and helped in writing the manuscript.
AA was a major contributor in writing the manuscript.
All authors read and approved the final manuscript.
Acknowledgements: We would like to acknowledge our great Kasr Al Ainy Hospital, and its workers, nurses and staff members, for all the support and help in this study and throughout our careers. Also acknowledge the great effort and sacrifice of the medical staff and nurses working during COVID-19 pandemic all over the world.
Ethical consideration: The study was approved by the institutional ethics committee and form review board of Hospital. Oral and written informed consent wasobtained from all subjects or from their eligible relatives. The medical record profession has its own code of ethics that applies to all medical record practitioners. Confidentiality of data, safe data storage, and privacy rights are respected by all who handle patient information. Data were coded and patient names or identity did not appear on any of the data collection forms or during statistical analysis.
References
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008; 117(25): 510-526. doi:10.1161/CIRCULATIONAHA.108.189141.
- Jaffe G, Gray Z, Krishnan G, et al. Screening Rates for Primary Aldosteronism in Resistant Hypertension: A Cohort Study. Hypertension. 2020; 75(3): 650-659. doi:10.1161/HYPERTENSIONAHA.119.14359.
- Libianto R, Fuller PJ, Young MJ, Yang J. Primary aldosteronism is a public health issue: challenges and opportunities. J Hum Hypertens. 2020; 34(7): 478-486. doi:10.1038/s41371-020-0336-2.
- Boulkroun S, Fernandes-Rosa FL, Zennaro MC. Molecular and Cellular Mechanisms of Aldosterone Producing Adenoma Development. Front Endocrinol (Lausanne). 2015; 6: 95. doi:10.3389/fendo.2015.00095.
- Drayer JI, Weber MA, Sealey JE, Laragh JH. Low and high renin essential hypertension: a comparison of clinical and biochemical characteristics. Am J Med Sci. 1981; 281(3): 135-142. doi:10.1097/00000441-198105000-00003.
- Calhoun DA, Booth JN 3rd, Oparil S, et al. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension. 2014; 63(3): 451-458. doi:10.1161/HYPERTENSIONAHA.113.02026.
- Adlin EV, Braitman LE, Vasan RS. Bimodal aldosterone distribution in low-renin hypertension. Am J Hypertens. 2013; 26(9): 1076-1085. doi:10.1093/ajh/hpt091.
- Ori Y, Chagnac A, Korzets A, et al. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol Dial Transplant. 2013; 28(7): 1787-1793. doi:10.1093/ndt/gfs587.
- Catena C, Colussi G, Sechi LA. Treatment of Primary Aldosteronism and Organ Protection. Int J Endocrinol. 2015; 2015: 597247. doi:10.1155/2015/597247.
- Chomsky-Higgins Menut K, Pearlstein SS, Conroy PC, et al. Screening for primary aldosteronism in the hypertensive obstructive sleep apnea population is cost-saving [published online ahead of print. Surgery. 2021; S0039-6060(21): 00552-3. doi: 10.1016/j.surg.2021.05.052.
- Corbin F, Douville P, Lebel M, et al. Active renin mass concentration to determine aldosterone-to-renin ratio in screening for primary aldosteronism. Int J Nephrol Renovasc Dis. 2011; 4: 115-120.
- Chan YH. Biostatistics 102: quantitative data--parametric & nonparametric tests. Singapore Med J. 2003; 44(8): 391-396.
- Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003; 44(12): 614-619.
- Gonzaga CC, Calhoun DA. Resistant hypertension and hyperaldosteronism. Curr Hypertens Rep. 2008; 10(6): 496-503. doi:10.1007/s11906-008-0092-0.
- Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline [published correction appears in J Clin Endocrinol Metab. 2021; 106(7): 2851. J Clin Endocrinol Metab. 2008; 93(9): 3266-3281. doi:10.1210/jc.2008-0104.
- Kline GA, Prebtani APH, Leung AA, Schiffrin EL. Primary aldosteronism: a common cause of resistant hypertension. CMAJ. 2017; 189(22): 773-778. doi:10.1503/cmaj.161486.
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016; 101(5): 1889-1916. doi:10.1210/jc.2015-4061.
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006; 48(11): 2293-2300. doi: 10.1016/j.jacc.2006.07.059.
- Tiu SC, Choi CH, Shek CC, et al. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005; 90(1): 72-78. doi:10.1210/jc.2004-1149.
- Harvey AM. Hyperaldosteronism: diagnosis, lateralization, and treatment. Surg Clin North Am. 2014; 94(3): 643-656. doi: 10.1016/j.suc.2014.02.007.
- Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004; 89(3): 1045-1050. doi:10.1210/jc.2003-031337.
- Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism. A systematic review of the literature. Endocrinol Metab Clin North Am. 2002; 31(3): 619. -xi. doi:10.1016/s0889-8529(02)00013-0.
- Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012; 44(3): 170-176. doi:10.1055/s-0031-1295460.