SciBase Journals
SciBase Critical Care and Emergency Medicine
ISSN 2691-7785
- Article Type: Research Article
- Volume 2, Issue 2
- Received: Mar 30, 2024
- Accepted: April 26, 2024
- Published Online: April 29, 2024
The Effect of Mesalazine on Male Mice Model with Ulcerative Colitis
Bo-Ran An1*; Yangyang Wang1; Jinging Wang2; Jiali Zhu1; Zhe Jia3
11st Ward, Gastroenterology Department, Affiliated Hospital of Hebei University, China.
2Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, China.
32nd Ward, Gastroenterology Department, Affiliated Hospital of Hebei University, China.
*Corresponding Author: Bo-Ran An
1st Ward, Gastroenterology Department, Affiliated Hospital of Hebei University, China.
Email: hbboran@sina.com
Abstract
Objective: To investigate the effect of mesalazine on Ulcerative Colitis (UC) in male mice model.
Method: Male mice aged 6-8 weeks were selected and randomly divided into Dextran Sodium Sulfate group (DSS group), mesalazine group (Tr group), and Control group (Con group). During the experiment, observe the body weight, stool characteristics, occult blood, changes in mice hair, eating habits and activity status, etc. After euthanizing the mice, eyeball blood was taken to measure IL-6 and TNF-α, and Disease Activity Index (DAI) was calculated. Take colon tissue (from the end of the cecum to the anal segment) and measure its length, and make paraffin sections, and observe them under a microscope after HE staining, and calculate their histopathological scores.
Result: On the 7th day, the DAI score in the DSS group was higher than that in the Con and Tr groups. The Tr group was higher than the Con group, and the differences were statistically significant. About weight changes, DSS group mice showed a decrease of over 15%. The final weight loss of Tr group mice was less than 10%. On the 8th day, there were differences in weight changes among the three groups of mice, and the differences were statistically significant. The differences in the levels of TNFαand IL-6 in the serum of each group were statistically significant. Pairwise comparison showed that DSS group was higher than Con group and Tr group, and the difference was statistically significant. The histopathlogical score in DSS group was higher than that in Con and Tr groups, and the histopathological score of Tr was higher than that of Con group, and the differences were all statistically significant. The length of the large intestine was shortened in DSS group than that in Con group and Tr group, and the colon length of Con group mice was significantly higher than that of Tr group mice.
Conclusion: Using 4% DSS solution, all indicators showed that the mice UC model was successfully established. Mesalazine can alleviate the symptoms of mouse UC model, and reduce the levels of some serum inflammatory factors, and improve its histopathological score, but it cannot block that DSS induced UC occurrence.
Keywords: Mice; Ulcerative colitis; Model; Mesalazine.
Citation: BR An, Wang Y, Wang J, Zhu J, Jia Z. The Effect of Mesalazine on Male Mice Model with Ulcerative Colitis. SciBase Crit Care Emerg Med. 2024; 2(2): 1008.
Introduction
In recent years, with the rising level of global industrialization, the incidence rate of UC has increased year by year in some countries, seriously affecting public health [1]. Therefore, research on UC is becoming increasingly urgent. Due to the experimental conditions can be controlled well in using animal models, it remains an important method to study UC with it. We successfully established male mice UC model using 4% DSS solution. In the experiment, intervention with mesalazine on UC mice failed to completely prevent colonic inflammation. We report it as follows.
Materials and methods
Animals, main reagents and instruments: 27 C75BL/6 inbred mice, 6-8 weeks old, weighing 18-20 g, purchased from Sun Yat sen University (Medical Experimental Animal Center). DSS was purchased from MP Biomedicals (United States), and the sustained-release granules of mesalazine (trade name: Adisa) were donated by 422 Hospital of the People’s Liberation Army of China. The ELISA kit for mice TNF-αand IL-6 assaying were purchased from Shanghai Yanhui Biotechnology Co. Ltd. The low-temperature high-speed centrifuge is produced by Thermolyn (United States).
Establishment of animal models of acute UC in mice: 27 male mice, aged 6-8 weeks were randomly divided into DSS induced model group (DSS group), control group (Con group), and mesalazine treated group (Tr group), with 9 mice in each. They were raised in a normal environment, and all groups ate regularly. The DSS group drank 4% DSS solution while the Con group was given common drinking water, and the Tr group received gavage administration of mesalazine granules on the first day of the experiment, in addition to drinking 4% DSS solution. Convert the body surface area of experimental animals to the equivalent dose ratio of the human body, and convert the drug dosage in the human body to equivalent dose of mice per day [2]. The Interventions on each group lasted for 7 days.
On the 8th day, mice were euthanized, and the length of the colon was measured. Eye blood and colon tissue (from the end of the cecum to the anus) were collected. Calculate the pathological score of colon tissue [3]. The serum was separated from eyeball blood at a low temperature of 3000 rpm, and then packaged and stored in an EP tube at -80OC for testing. Use an ELISA detection kit to determine the levels of TNF-αand IL-6 in mouse serum.
During the experiment, measure the daily weight of mice and calculate their DAI.
Statistical processing
Statistical analysis software SPSS19.0 was used for analysis. For the measurement data of normal distribution, the results are represented by the mean standard deviation ( x s ± ). Perform a homogeneity test of variance on each group of measurement data. When the variance is homogeneous, t-tests or analysis of variance are used for inter group comparisons. If the variance is uneven, Welch’s approximate analysis of variance is used. The comparison of DAI among the three groups was conducted using Kruskal Wallis H test.
Results
General observations
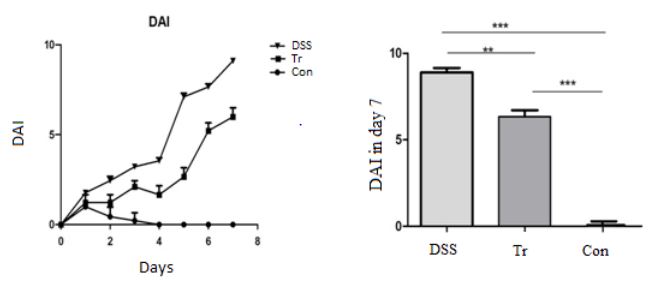
During the experiment, the Con group mice showed normal water and food intake, shiny hair, sensitive reactions, formed stools, and negative occult blood test. The DSS group mice could drink 4% DSS solution freely as the experiment began. On 3rd day, the mice in DSS group began to experience reduced eating, arched back, easily startled, dull hair, slow response to stimulate, positive fecal occult blood test. By the 5th day, the movement of them reduced, and varying degrees of bloody stools were spot. In severe cases, mice experienced anal bleeding, significant weight loss, and messy hair. By the 8th day, their weight had decreased by more than 15%. The weight changes and mouse symptoms in the Tr group were between those in the Con and DSS groups. The DAI scoring criteria are shown in Table 1. The scoring chart is shown in Figure 1. In Figure 1, the left shows the trend of changes in DAI scores among the three groups of mice, while the right shows a comparison of DAI scores among the three groups of mice on the 7th day. On the 4th day, the DSS group mice showed visible mucus and bloody stools in naked eyes. The stool OB test was positive, and the mice showed inactive to stimulate and dull hair. However, the Con group mice showed normal activity without significant changes compared to the beginning of the experiment, and no occult blood was detected in the stools. Comparing the DAI scores of the three groups of mice on the 7th day, it was found that the DSS group was significantly higher than the Con group, with a statistically significant difference (P<0.001). The DSS group was higher than the Tr group, with a statistically significant difference (P<0.01).
Table 1: DAI score (Daily Disease Activity Index).
| Score | Weight loss (%) |
Stool character | OB |
|---|---|---|---|
| 0 | 0 | Normal | - |
| 1 | 1-5 | soft but formed | + |
| 2 | 5-10 | very soft | (++~+++)or blood can be seen in the stool |
| 3 | 10-15 | Diarrhea | rectal and anal bleeding |
| 4 | >15 |
Table 2: Comparison of body weight changes among three groups of mice ( x s± ).
| Groups | N | Before experiment | Day 4 | Day 8 |
|---|---|---|---|---|
| Con | 9 | 20.41 ± 0.47 | 21.28 ± 0.51 | 22.20 ± 0.64 |
| Tr | 9 | 20.24 ± 0.38 | 20.1 ± 0.40 | 18.89 ± 1.42△* |
| DSS | 9 | 20.32 ± 0.0.47 | 18.77 ± 0.51 | 15.18 ± 0.82** |
Table 3: Serum TNF-α and IL-6 concentrations in three groups of mice ( x±s).
| Group | N | TNF-α(pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|
| Con | 9 | 32.19 ± 1.55 | 30.46 ± 3.91 |
| Tr | 9 | 34.67 ± 2.03△* | 38.74 ± 4.75△▲ |
| DSS | 9 | 47.67 ± 1.32** | 61.58 ± 4.47** |
Note: *represents Tr VS Con P<0.05, **represents DSS VS Con P<0.001, △ represents DSS VS Tr P<0.001, ▲ represents Tr VS Con P<0.001
Comparison of body weight changes among three groups of mice
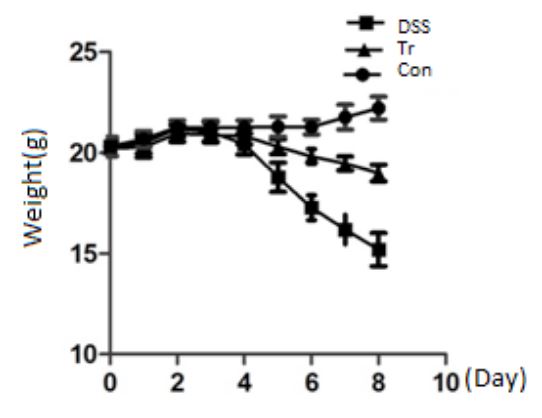
Figure 2 shows the trend of weight changes in DSS group, Con group, and Tr group mice. Table 2 shows the weight changes of the three groups of mice before experiment and on the 4th and 8th days of experiment. The results showed that there was no statistically significant difference in initial body weight among the three groups of mice (P>0.05). During the experiment, the weight of the Con group mice showed an upward trend. The DSS group mice began to experience weight loss on the 4th day, and by the 8th day, their weight decreased by more than 15% compared to the begining of experiment. The weight loss of Tr group mice was slower than that of DSS group, and the final weight loss was less than 10%. On the 8th day, there were differences in weight changes among the three groups of mice, and the differences were statistically significant (P<0.001).
2.3 Mice serum TNF-α and IL-6 is shown in Table 3. The difference in the content of TNF-α in the serum of each group was statistically significant (P<0.05). Pairwise comparison showed that the content of TNF-α in the serum of the DSS group was significantly higher than that of the Con and Tr groups, with a statistically significant difference (P<0.001). The differences in the levels of IL-6 in the serum of each group were statistically significant. Pairwise comparison showed that the serum IL-6 content in the DSS group was significantly higher than that in the Con and Tr groups, with a statistically significant difference (P<0.001). The Tr group was higher than the Con group, and the difference was statistically significant (P<0.001).
The pathological scores of mouse colon tissue are shown in Figures 3 and 4.
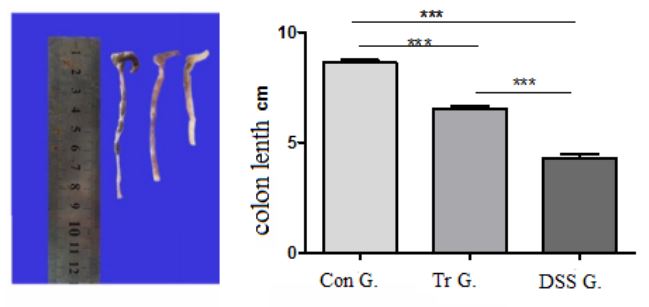
Pathological general observation
After euthanizing the mice on the 8th day, the colon tissue (from the anus to the end of the cecum) was taken for observation. The results are shown in Figure 3. The Con group mice had the longest colon length, smooth colonic mucosa, no erosion, congestion, edema, and no obvious damage. The colon of DSS group mice showed congestion and edema, with visible erosion, ulcers, and bleeding in the mucosa. Compared with the control group, the length of the colon was significantly shortened, and the difference was statistically significant (P<0.001). The colon mucosal damage in Tr group mice was lighter than that in DSS group mice, and the colon length was longer than that in DSS group mice, with a statistically significant difference (P<0.001). The colon length of Con group mice was significantly higher than that of Tr group mice (P<0.001).
Pathological score
Take pathological sections of colon tissue from each mouse and read them using blind method. The total score=Σ(items points×Lesion range). Calculate the pathological score according to follow:
0 score: no Integral inflammatory cell infiltration. No crypt rupture. Complete regeneration or normal. Lesion range, 0%. 1 score: inflammatory cell dispersion (1-2 foci) (mild). 1/3 crypt rupture, basal layer is almost completely regenerated. Lesions range, 1-25%. 2 score: multiple lesions, moderate. Basal layer 2/3 regeneration with crypt defects. Lesions range, 25-50%. 3 score: Vascular congestion, mucosal thickening (severe). Total crypt inflammation, only epithelial lining remains. Surface epithelium incomplete regenerated. Lesions range, 50-75%.4 score: Wall permeability, loss of goblet cells (extremely severe). Total crypt and epithelial loss. No repair. Lesions range, 75- 100%.
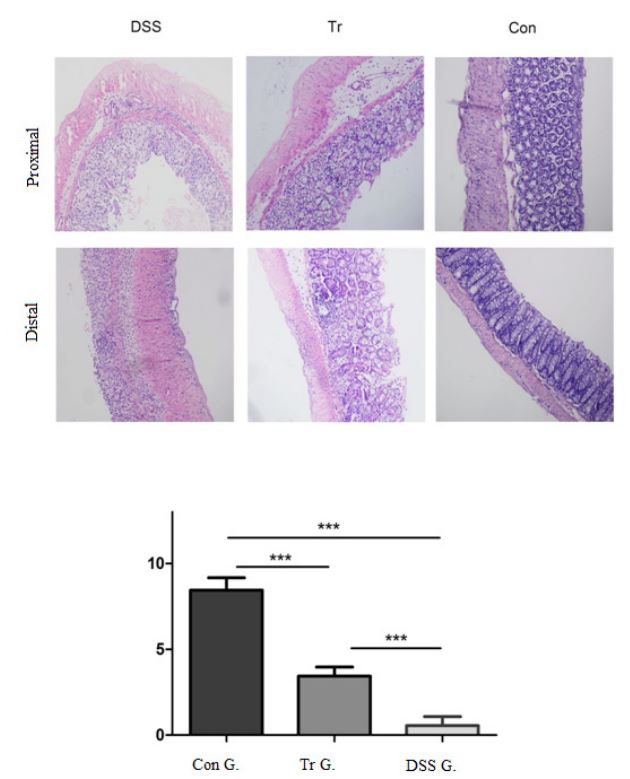
Electronic microscopy was employed to observe HE staining sections and to perform pathological scoring on mouse colon tissue. The results are shown in Figure 4. The colon mucosa in Con group mice are continuous and intact, with mucosal glands arranged neatly, and no damage to crypt structure, no inflammation or ulcer. The mucosal tissue structure of DSS group mice is disordered, with incomplete mucosa, disappearance of crypt structure, visible ulceration, and a large number of inflammatory cells infiltrating the mucosa and submucosa. The degree of tissue structure damage in the Tr group was relatively mild, and glandular disorders and mucosal damage were also observed, but the infiltration of inflammatory cells was less than that in the DSS group. The histopathological score of the DSS group was higher than that of the Con and Tr groups, and the differences were statistically significant (P<0.001). The histopathological score in Tr group was higher than that of the Con group, and the difference was statistically significant (P<0.001).
Discussion
UC is a disease with unknown causes, which is currently believed to be related to genetic factors, infections, immune factors, environmental factors, etc. When studying UC, due to the controllable experimental conditions of animal models, it is still a reliable method for studying UC. DSS, as an inflammatory substance, can cause inflammation of the colon mucosa and is still widely used in the making of animal models of UC, and the literature records show that 0.5%-5% DSS solution is often used to make it [4]. We used 4% DSS solution to make UC model, which made it easier to establish, and there was no mouse death during the experiment. Using mice of a single male gender, the same group can be raised together, which can reduce experimental costs.
By observing the DAI index, body weight, IL-6 and TNF-α, and histopathological scores of each group of mice, it was indicated that the UC animal model of the mice was successfully established. In the Tr group, we administered Mesalazine to mice by gavage at the beginning of the experiment, while the mice drank DSS solution simultaneously, it can still lead to UC in them, indicating that Mesalazine cannot completely inhibit inflammatory reactions.
Conclusion
4% DSS concentration is good for making mice UC model of which is easier while mice mortality will be lower during the experiment. It is suggested that in clinical treatment of UC, pathogenic factors should be removed while applying therapeutic drugs.
Declarations
Data availability: The data are available from the corresponding author upon reasonable request.
Competing interests: The authors declare that they have no conflicts of interest.
Funding: Supported by General project, funded by the Natural Science Foundation of Hebei Province (2023). Project number: H2023201022
Acknowledgements: We are acknowledge 422 Hospital of PLA for having donated Mesalazine sustained-release granules which was used in the experiment.
References
- Yamazaki M, Chung HW, Xu YR, et al. Trends in the prevalence and incidence of ulcerative colitis in Japan and the US. Int J Colorectal Dis. 2023; 38: 135. doi: 10.1007/s00384-023-04417-6.
- Guidelines for Operating Common Animal Experiments[M]. Zhang Bin,Wang Yuhui.Shanghai University of Traditional Chinese Medicine Press(Chinese). 2007: 195-199.
- Koller FL, Dozier EA, Nam KT, et al. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab Invest. 2012; 92: 1749- 1759.
- Vicario M, Crespí M , Franch A, et al.Induction of colitis in young rats by dextran sulfate sodium. Dig Dis Sci. 2005; 50: 143-150.