SciBase Journals
SciBase Critical Care and Emergency Medicine
ISSN 2691-7785
- Article Type: Research Article
- Volume 2, Issue 2
- Received: Apr 15, 2024
- Accepted: Jun 29, 2024
- Published Online: Jul 05, 2024
Carboxyhemoglobin in Critically Ill Septic Patients
Atthaphong Phongphithakchai1,2*; Akinori Maeda1 ; Yukiko Hikasa1 ; Sofia Spano1 ; Nuttapol Pattamin1 ; Anis Chaba1 ; Glenn Eastwood1,3,4; Helen Young1 ; Leah Peck1 ; Rinaldo Bellomo1,3,4,5,6
1Department of Intensive Care, Austin Hospital, Melbourne, VIC, Australia.
2Department of Internal Medicine, Division of Nephrology, Faculty of Medicine, Prince of Songkhla University, Hat Yai, Songkhla, Thailand.
3Department of Critical Care, the University of Melbourne, Melbourne, VIC, Australia.
4Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne, VIC, Australia.
5Data Analytics Research and Evaluation Centre, Austin Hospital, Melbourne, Australia.
6Department of Intensive Care, Royal Melbourne Hospital, Melbourne, Australia.
*Corresponding Author: Atthaphong Phongphithakchai
Department of Intensive Care, Austin Hospital, Melbourne, VIC, Australia.
Tel: +61-3-9496-5992 & +61-3-94963932;
Email: ton331@hotmail.com & tony.phongphithakchai@austin.org.au
Abstract
Background: Sepsis-associated prognostic biomarkers are potentially useful for patient selection and stratification into sepsis trials. An elevated Carboxyhemoglobin (COHb) reflects heme degradation and, indirectly, red cell injury and is readily and freely available with Arterial Blood Gases (ABGs) measurements.
Aim: To test the hypothesis that COHb would be a useful prognostic marker in patients with sepsis.
Methods: We performed an observational cohort study of all adult patients diagnosed with sepsis who were admitted to our ICU between 2016 to 2020 Using the peak COHb levels during the first 24-hours of ICU admission we explored the i) epidemiology of peak COHb levels and the association of COHb with sepsis outcomes and ii) its correlation with relevant biochemical and clinical variables.
Result: Among of 1,368 septic patients, 873 patients (64%) had complete data and met the inclusion criteria. The median peak COHb level was 1.5% and patients with COHb levels ≥1.5% had higher illness severity scores, higher bilirubin levels and lower haematocrit levels (P<0.001). When COHb was divided into quartiles, the highest quartile had a greater incidence of patients with cirrhosis (P<0.001), higher illness severity scores (P=0.003), a higher bilirubin level (P<0.001) lower hematocrit levels (P<0.001) levels. In such patients, ICU and hospital length of stay was significantly longer but in-hospital mortality was similar. On multivariable analysis, gender, Acute Physiology and Chronic Health Evaluation (APACHE) II score, congestive heart failure, and hematocrit levels had significant independent relationships with COHb levels.
Conclusion: Elevated COHb levels within the first 24 hours of ICU admission are associated with higher bilirubin levels and lower hematocrit levels and predict longer ICU and hospital stay but not increased mortality. These findings suggest that COHb has limited value as an outcome biomarker in sepsis.
Keywords: Sepsis; Carboxyhemoglobin; Heme oxygenase-1; ICU mortality.
Citation: Phongphithakchai A, Maeda A, Hikasa Y, Spano S, Pattamin N, et al. Carboxyhemoglobin in Critically Ill Septic Patients. SciBase Crit Care Emerg Med. 2024; 2(2): 1010.
Introduction
Sepsis remains one of the most challenging conditions within the Intensive Care Unit (ICU) and is characterized by a dysregulated host response to infection leading to organ dysfunction [1]. Because it is associated with a high mortality rate, rapid diagnosis and treatment are important [2]. Thus, diagnostic or prognostic biomarkers may help clinicians to aid in the identification and stratification of patients into sepsis trails aimed to improve outcomes [3,4].
Heme Oxygenase-1 (HO-1) plays a significant role in the degradation of heme into biliverdin, iron, and carbon monoxide (CO) [5,6]. This enzyme, inducible by factors such as hyperoxia, hypoxia, bacterial lipopolysaccharides, and cytokines [7,8] and is upregulated in sepsis, leading to increased production of CO [9,10]. Such CO then binds to hemoglobin forming carboxyhemoglobin (COHb) which is easily measured with Arterial Blood Gases (ABGs) and may serve as an indirect marker of HO-1 activity and inflammation severity (Figure S1) [6]. Some studies have shown a significant increase in COHb levels associated with lateonset sepsis in preterm infants, whereas others have found no significant relationship between the onset of sepsis and COHb levels in this population [8,11]. Thus, the utility of COHb as biomarker in sepsis is unclear.
Point-of-care Arterial Blood Gas (ABG) measurement now offers that ability to easily measure COHb levels, [12] in routine care and investigate COHb in greater detail. Thus, our primary aim was to evaluate the epidemiology of peak COHb during the first 24 hours of ICU admission in critically ill septic patients including role of COHb as a predictor of sepsis outcome in ICU and test the primary hypothesis that COHb is a predictor of mortality is sepsis. The secondary objectives were to determine the correlation between COHb and relevant biochemical and clinical variables and to identify independent predictors of COHb in septic patients.
Materials and methods
Study design and population
This was a single center retrospective observational cohort study conducted at the Austin Hospital, a tertiary hospital affiliated with the University of Melbourne, in Melbourne, Australia. From July 2016 to June 2020, we included all adult patients admitted to the Intensive Care Unit (ICU) who met the criteria for sepsis as defined by the Third International Consensus Definitions for Sepsis and Septic Shock (SEPSIS-3). The study population needed to exhibit two specific criteria: 1) signs of infection as determined by an admission diagnosis aligning with sepsis or infection according to the Acute Physiology and Chronic Health Evaluation (APACHE) III and 2) signs of organ dysfunction, evidenced by an increase of 2 or more points in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score within the first 24 hours of ICU admission.
The criteria for sepsis definition and APACHE diagnosis can be found in the Supplementary Methods. Exclusions were made for patients transferred to another hospital and those with incomplete data, including absent COHb measurements or unrecorded ICU or hospital discharge status. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendation guidelines [13]. Approval was granted by the Austin Health Human Research Ethics Committee (HREC/97457/Austin-2023). The requirement for written informed consent was waived due to the data being analyzed in an anonymized manner.
Data source and collection
The data source included from both the Australia New Zealand Intensive Care Society (ANZICS) Australian Patient Database (APD) hosted by Austin Hospital and electronic medical records. Demographic data were obtained for patients in the first 24 hours of ICU admission including age, gender, and comorbidities. APACHE II, APACHE III and SOFA score were calculated at initial admission. Clinical parameters and laboratory parameters were also collected as followed: Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), urine output, hematocrit, platelet count, total bilirubin, urea, Creatinine (Cr), arterial blood gas analysis including partial Pressure of Oxygen (PaO2 ) and lactate, vasopressors use and mechanical ventilation use. Finally, we collected information on length of stay (LOS) in the ICU or hospital and survival status at hospital discharge.
COHb levels in first 24 hours of ICU admission were determined by an arterial blood gas analyzer (ABL 800FLEX, Radiometer, Copenhagen, Denmark) calibrated with internal reference standards and results was automatically transferred to the electronic laboratory medical records. Arterial blood COHb levels were analyzed through derivative spectrophotometry using an automated CO-oximeter, which offers a range of 0-100% COHb measurement with an accuracy of ±0.5% [14]. COHb levels were presented as a percentage of the total Hb content. In cases where patients had several COHb results within the first 24 hours after being admitted to the ICU, the highest level was selected for analysis.
Statistical analysis
Data were analysed using R version 4.2.1 (R Core Team. 2022 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Viena Austria). Categorical variables were summarized as frequencies and percentages per column, whereas continuous variables were detailed by either mean and Standard Deviation (SD) or median alongside the 25th and 75th quartiles. The comparison of categorical variables was performed using the Chi-square test or Fisher’s exact test. Continuous variables were analysed through the t-test or MannWhitney U-test, selecting the test based on the variable type and data distribution. A correlation analysis between COHb and other relevant variables (total bilirubin and blood lactate) was performed. Based on non-parametric distribution of data, the Spearman correlation test was used.
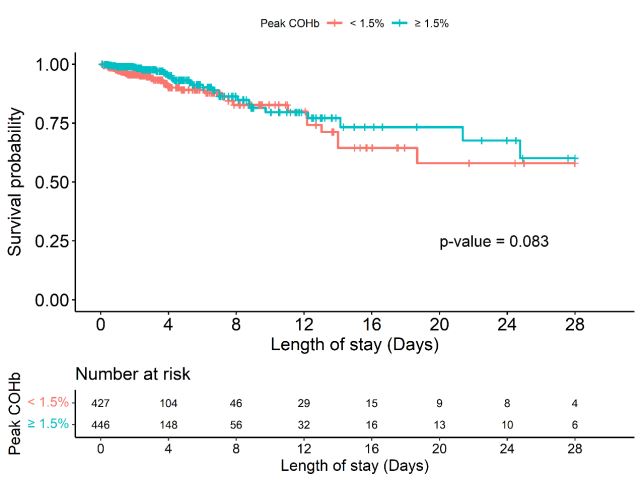
A Kaplan-Meier curve was used to present the 28-days hospital mortality among patients based on their those with COHb levels above and below the median value. This comparison utilized the log-rank test to assess statistical significance. The relationship between peak COHb and other variables including gender, age, comorbidities, clinical parameters, and laboratories parameter was assessed through univariate and multivariate linear regression models. Variables that achieved a P-value of less than 0.2 were considered for inclusion in the multivariate models. Factors related to 28-days hospital mortality were evaluated using univariate and multivariate logistic regression, reported as Odds Ratios (OR) with 95% confidence intervals (CI). A p-value of less than 0.05, within a 95% confidence interval, was deemed to indicate statistical significance.
Results
Between July 2016 and June 2020, our hospital admitted 1,368 adult patients who fulfilled the sepsis criteria. Of these, 495 patients were not considered for the final analysis due to significant missing data (n=476) and being transferred to another hospital (n=19) leaving 873 patients for analysis. As shown in Table 1, their median age was 63.8 years and 59.7% were male. The most common comorbidities were chronic kidney disease and chronic lung disease. The median APACHE III, and SOFA score were 60 and 7, respectively. More than a half of patients were on vasopressors (53.2%) and mechanical ventilation (71.8%). The median peak COHb was 1.5%. The characteristics of patients divided according to who had above (≥1.5%) or below (<1.5%) the median peak COHb for the entire cohort are shown in Table 1. Those with COHb above the median were more likely to be male, have cirrhosis, and higher illness severity scores. Bilirubin levels were significant higher and hematocrit levels significantly lower in these population.
We also categorized the patients by the quartile of peak COHb as illustrated in Table 2. In keeping with the findings above, patients in the highest COHb quartile were also more likely to be male, have a higher prevalence of cirrhosis, higher illness severity scores, higher bilirubin levels and lower hematocrit concentration. In addition, they had significantly lower diastolic blood pressure levels.
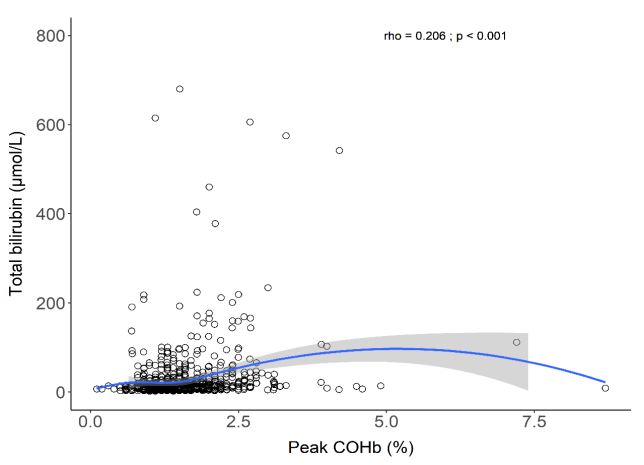
Patients with peak COHb levels ≥1.5% had significantly longer ICU stays compared to the <1.5% group (2.7 days vs. 2.3 days, p<0.001) and hospital stays (15.8 days vs. 10.6 days, p<0.001). However, there was no significant difference in in-hospital mortality between the two groups (Table 1). Similarly, when patients were divided by quartiles, those in higher quartiles experienced longer ICU and hospital stays, but mortality rates did not differ (Table 2). Figure 1 presents the Kaplan-Meier curve showing a non-significant difference in time to 28-days hospital mortality between patients according to a COHb level above or below the median. There was a statistically significant positive correlation between peak COHb and total bilirubin (rho=0.206, p<0.001) (Figure 2). However, we found no correlation between peak COHb and other variables including blood lactate and PaO2 (Figure S2 and Figure S3).
As shown in Table 3, multivariable regression analysis for 28-day mortality revealed only three independent predictors including APACHE II score (OR: 1.16; 95% CI: 1.08-1.24), vasopressor use (OR 2.80; 95% CI: 1.28-6.13) and lactate level (OR: 1.32; 95% CI: 1.15-1.50). As shown in Table 4, multiple linear regression analysis for peak COHb found a positive association between COHb and congestive heart failure (β=0.153, p=0.011) and APACHE II score (β=0.012, p=0.007) and a negative association between peak COHb and female sex (β=-0.196, p<0.001) and hematocrit levels (β=-0.019, p<0.001).
Table 1: Baseline characteristics and clinical outcomes of patients with peak COHb ≥1.5% versus <1.5% in first 24 hours.
| Overall (N=873) | Peak COHb ≥1.5% (N=446) | Peak COHb <1.5% (N=427) | P value | |
|---|---|---|---|---|
| Age | 63.8(51.6-74.8) | 63.3(51.7-73.1) | 65(51.3-75.9) | 0.22 |
| Gender- | N | (%) | 0.017 | |
| Female | 352(40.3) | 162(36.3) | 190(44.5) | |
| Male | 521(59.7) | 284(63.7) | 237(55.5) | |
| COPD | 130(14.9) | 69(15.5) | 61(14.3) | 0.692 |
| Congestive heart failure | 49(5.6) | 28(6.3) | 21(4.9) | 0.468 |
| Cirrhosis | 74(8.5) | 56(12.6) | 18(4.2) | <0.001 |
| CKD | 160(18.3) | 92(20.6) | 68(15.9) | 0.088 |
| SBP, mmHg | 135(122-150) | 135(124-150) | 135(122-150) | 0.735 |
| DBP, mmHg | 65(58-75) | 65(58-75) | 65(58-77.5) | 0.237 |
| Urine output, mL/day | 1522(961-2273) | 1528(988-2246) | 1510(961-2278) | 0.990 |
| APACHE II score | 18(13-22) | 18(14-22) | 17(12-22) | 0.014 |
| APACHE III score | 60(43-75) | 62(45-76) | 58(41.5-73.5) | 0.057 |
| SOFA score | 7(5-10) | 8(5-10) | 7(5-10) | 0.032 |
| Hematocrit level, L/L | 0.3(0.3-0.4) | 0.3(0.2-0.4) | 0.3(0.3-0.4) | <0.001 |
| Platelet count, x109/L | 203(131-289) | 196(122-280) | 212(139-302) | 0.073 |
| Total bilirubin, μmol/L | 13(8-25) | 14(9-32) | 11(7-20) | <0.001 |
| Urea level, mmol/L | 9.9(5.8-17.7) | 10.6(6.3-18.2) | 9.6(5.4-16.1) | 0.075 |
| Creatinine level, μmol/L | 120(77.5-218) | 127(79-247) | 113(76-200) | 0.115 |
| Arterial blood gas | ||||
| pH | 7.4(7.3-7.4) | 7.4(7.3-7.4) | 7.4(7.3-7.4) | 0.017 |
| PaO2 | 76(65-101) | 73(64-100) | 78(65-106) | 0.077 |
| PaO2/FiO2 | 285(173-376) | 281(175-371) | 296(172-376) | 0.639 |
| Lactate level, mmol/L | 2.2(1.5-3.2) | 2.2(1.5-3.1) | 2.2(1.5-3.5) | 0.290 |
| Vasopressor uses | 464(53.2) | 233(52.2) | 231(54.1) | 0.630 |
| Mechanical ventilation | 516(71.8) | 271(73.8) | 245(69.6) | 0.238 |
| ICU LOS in days | 2.6(1.5-4.6) | 2.7(1.7-4.8) | 2.3(1.2-4) | <0.001 |
| Hospital LOS in days | 13.2(6.6-27.5) | 15.8(7.3-33.3) | 10.6(5.9-21.6) | <0.001 |
| In-hospital mortality | 105(12) | 52(11.7) | 53(12.4) | 0.812 |
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; CKD: Chronic Kidney Disease; CI: Confidence In- terval; DBP: Diastolic Blood Pressure; Pao2/Fio2: The Ratio of Partial Pressure of Oxygen to the Fraction of Inspired Oxygen; SBP: Systolic Blood Pressure; SOFA: Sequential Organ Failure Assessment. Data presented in number (%) or median (IQR).
Table 2: Baseline characteristics and clinical outcomes of patients with peak COHb stratified by quartiles in first 24 hours.
| Q1 (N=203) | Q2 (N=224) | Q3 (N=228) | Q4 (N=218) | P value | |
|---|---|---|---|---|---|
| Age | 63.1(50.5-75.6) | 66(53.3-77.4) | 63.9(50.6-74) | 62(53-71.4) | 0.254 |
| Gender- N (%) | 0.029 | ||||
| Female | 109(53.7) | 81(36.2) | 86(37.7) | 76(34.9) | |
| Male | 94(46.3) | 143(63.8) | 142(62.3) | 142(65.1) | |
| COPD | 178(87.7) | 188(83.9) | 191(83.8) | 186(85.3) | 0.651 |
| Congestive heart failure | 8(3.9) | 13(5.8) | 9(3.9) | 19(8.7) | 0.110 |
| Cirrhosis | 6(3) | 12(5.4) | 19(8.3) | 37(17) | <0.001 |
| CKD | 33(16.3) | 35(15.6) | 52(22.8) | 40(18.3) | 0.193 |
| SBP, mmHg | 134(120-150) | 138(125-151) | 137(125-150) | 132(120-149) | 0.568 |
| DBP, mmHg | 70(60-80) | 65(55-75) | 65(60-77.8) | 62(55-75) | 0.007 |
| Urine output, mL/day | 1392(957-2048) | 1687(994-2536) | 1515(827-2234) | 1555(1085-2250) | 0.125 |
| APACHE II score | 17(12-21) | 17(13-22) | 18(12.5-21.5) | 19(15-23) | 0.003 |
| APACHE III score | 58(42-77) | 58(41-73) | 59(43-72) | 64(48-79) | 0.007 |
| SOFA score | 7(5-10) | 7(5-9) | 7(5-9) | 8(5-10) | 0.015 |
| Hematocrit level, L/L | 0.3(0.3-0.4) | 0.3(0.3-0.4) | 0.3(0.3-0.4) | 0.3(0.2-0.3) | <0.001 |
| Platelet count, x109/L | 215(148-304) | 210(134-299) | 216(142-289) | 184(111-263) | 0.095 |
| Total bilirubin, μmol/L | 11(7-19) | 11.5(7-20.8) | 12(8-23) | 18(9.5-44) | <0.001 |
| Urea level, mmol/L | 9.2(5.3-16.1) | 9.8(5.7-16.1) | 10.1(6.2-17) | 10.9(6.7-18) | 0.196 |
| Creatinine level, μmol/L | 109(72-206) | 120(82-196) | 124(74-227) | 131(85-251) | 0.265 |
| Arterial blood gas | |||||
| pH | 7.4(7.3-7.4) | 7.4(7.3-7.4) | 7.4(7.3-7.4) | 7.4(7.4-7.5) | 0.090 |
| PaO2 | 79(66-107) | 78(65-105) | 72(66-97) | 75.5(63-101) | 0.366 |
| PaO2/FiO2 | 296(171-376) | 295(173-377) | 278(172-361) | 281(181-374) | 0.950 |
| Lactate level, mmol/L | 2.2(1.5-3.4) | 2.2(1.5-3.6) | 2.2(1.6-3.1) | 2.2(1.5-3.1) | 0.697 |
| Vasopressor use | 99(48.8) | 132(58.9) | 121(53.1) | 112(51.4) | 0.184 |
| Mechanical ventilation | 109(65.3) | 136(73.5) | 134(73.6) | 137(74.1) | 0.208 |
| ICU LOS in days | 2.3(1.1-4.7) | 2.2(1.2-3.8) | 2.7(1.6-4.6) | 2.8(1.7-4.9) | 0.008 |
| Hospital LOS in days | 11.5(5.9-25.8) | 10.4(5.9-17) | 14.6(7-29) | 17.9(8-36) | <0.001 |
| In-hospital mortality | 24(11.8) | 29(12.9) | 21(9.2) | 31(14.2) | 0.409 |
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; CKD: Chronic Kidney Disease; CI: Confidence Interval; DBP: Diastolic Blood Pressure; Pao2/Fio2: The Ratio of Partial Pressure of Oxygen to the Fraction of Inspired Oxygen; SBP: Systolic Blood Pressure; SOFA: Sequential Organ Failure Assessment. Data Presented In Number (%) or Median (IQR).
Table 3: Univariate and multivariate logistic regression models for variables influencing 28-day mortality.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| ORs | 95% CI | P value | ORs | 95% CI | P value | |
| Age > 60 years | 1.24 | 1.13-1.46 | 0.069 | |||
| APACHE II score | 1.92 | 1.10-3.36 | <0.001 | 1.16 | 1.08-1.24 | <0.001 |
| Congestive heart failure | 1.31 | 1.22-1.43 | 0.138 | |||
| Urine output | 1.81 | 1.15-3.36 | 0.027 | |||
| Creatinine level | 1.03 | 1.02-1.08 | 0.144 | |||
| Vasopressor use | 5.77 | 2.96-11.26 | 0.007 | 2.80 | 1.28-6.13 | 0.009 |
| Lactate level | 1.42 | 1.28-1.57 | <0.001 | 1.32 | 1.15-1.5 | <0.001 |
| Mechanical ventilation | 3.30 | 2.04–5.34 | <0.001 | |||
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; CI: Confidential Interval; Methb: Methemo- globin; Ors: Odd Ratios.
Table 4: Multivariable linear regression analysis with peak COHb as a dependent variable.
| Variables | β | SE | t | 95% CI | P value |
|---|---|---|---|---|---|
| Female | -0.196 | 0.054 | -3.595 | -0.300, -0.090 | <0.001 |
| Congestive heart failure | 0.153 | 0.112 | 1.340 | 0.007, 0.392 | 0.011 |
| APACHE II score | 0.012 | 0.004 | 2.706 | 0.000, 0.020 | 0.007 |
| Hematocrit level | -0.019 | 0.003 | -4.827 | -0.036, -0.015 | <0.001 |
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; β: Regression Coefficient; CI: Confiden- tial Interval; SOFA: Sequential Organ Failure Assessment.
Discussion
Key findings
In our single-center retrospective cohort study we evaluated the epidemiology of peak COHb in first 24-hours of ICU admission in critically ill septic patients to establish whether COHb can be useful prognostic marker. We had four key findings. First, patients with a highest COHb greater than 1.5% (the median) were more likely to be male and had a significantly higher severity scores and prevalence of cirrhosis, higher levels of total bilirubin and lower hematocrit values. These findings were confirmed by quartile analysis. Second, there was a significant prolongation of both ICU and hospital stay with higher COHb levels and a significant positive correlation between peak COHb and total bilirubin. Third, peak COHb were positively associated with congestive heart failure, higher APACHE II score, and hematocrit level and negatively associated with female sex and hematocrit levels. Finally, the APACHE II score, vasopressor use, and lactate levels serve as independent predictors of 28-day mortality.
Relationship with previous studies
HO-1 is the rate-limiting enzyme in the degradation of heme, which is involved in the inflammatory stress response pathway and protects cells from oxidative injury [15,16]. Detecting HO-1 in routine practice is not possible. However, measuring the indirect products of HO-1 activity (e.g., COHb) could be useful in sepsis as endogenous CO production by infrared CO analyzer has been detected in exhaled gas in septic shock patients [17]. In our study, we reported a median peak COHb level above 1.5% in the sepsis group. This finding aligns with a study on pediatric sepsis, which reported an average initial COHb of approximately 1.4% [18]. Furthermore, Grigorescu BL et al. [19]. Noted that adult patients, averaging 64 years old with bacterial sepsis, had a median COHb of 1.8% upon ICU admission. These findings highlight that, despite the known normal COHb levels of <2-5% in the general population, using such high cutoff points may not be appropriate in critically ill sepsis patients [20]. This discrepancy is likely due to a different underlying mechanism of COHb elevation, where increased COHb in ICU may reflect endogenous HO-1 activity, rather than exposure to exogenous CO.
We observed that patients with COHb levels above the median had higher bilirubin levels and that COHb showed a positive correlation with total bilirubin levels, in line with previous study in sepsis [19]. We speculate that this correlation arises from moderate activation of HO-1 activity, leading to a mild increase in both COHb and total bilirubin levels [21]. The higher prevalence of cirrhosis in patients with COHb levels greater than 1.5%, however, suggests that this relationship may well be confounded by the presence of liver disease.
We did not find an association between elevated COHb levels and in-hospital mortality. We hypothesize that this apparent contradiction could be explained by the level of HO-1 induction serving as an indicator of the disease severity and protection from adverse outcomes. Our cohort had an average APACHE II score of 18, similar that of a previous study that also found no correlation between COHb levels and estimated mortality [19]. However, Melley et al. [22]. reported that high COHb levels were associated with ICU mortality among cardiothoracic patients [23].
Clinical implications
Our findings imply that, in sepsis, the median peak COHb in the first 24 hours of ICU admission is only 1.5%. They also imply that patients with a levels about 1.5% and/or in the top quartile of COHb have greater illness severity, higher bilirubin levels, and lower hematocrit levels. Moreover, they suggest peak COHb can be used to identify patients with longer ICU and hospital stay. Finally, however, they show that COHb does not help predict increased mortality and is not a robust and clinically useful biomarker for patient selection or risk stratification of sepsis trials.
Strengths and limitations
Our study had a several strengths. The selection of study participants was based on accurate information as recorded in the ANZICS Adult Patient Database. Furthermore, the adoption of APACHE diagnosis coding for infections and aligning with SEPSIS-3 guidelines, improved the precision in identifying septic patients. Moreover, relying on initial laboratory values (first 24 hours) minimized the risk of exposure to potential therapies and interventions that could introduce confounding variables. Finally, it provides important information on the limitations of COHb as biomarker in sepsis.
We acknowledge several limitations. First, this is retrospective observational cohort analysis, inherently subject to its recognized limitation, including unmeasured confounding factors. Being conducted at a single center, it also has limitations typical of such designs, including limited generalizability. Nonetheless, our ICU has features common to other ICUs in similarly affluent countries, suggesting that our results may be applicable to comparable ICUs elsewhere. Second, sepsis was identified exclusively within the first 24 hours following ICU admission, resulting in the exclusion of patients who developed sepsis later during their ICU stay. Consequently, a smaller cohort of patients were enrolled in the study. However, this methodology allowed the selection of a precisely defined population based on wellestablished sepsis criteria. Third, while we hypothesize that elevated COHb levels result from HO-1 induction in sepsis, we face the challenge of not having established normal COHb ranges for critically ill patients and being unable to measure HO-1 activity. This lack of data limits the biological plausibility of our hypothesis. However, based on the study by Maeda A et al. [24]. Which found that pre-operative COHb levels in most cardiothoracic critically. ill patients did not exceed 1%, our findings suggest a greater HO-1 activation in sepsis. Fourth, we measured COHb levels using the blood CO-oximetry method to reflect the endogenous CO content. However, this method is not the most accurate or reliable for precise COHb level determination. Gas chromatography offers more accuracy in measuring COHb [25,26]. Nevertheless, this technique is less practical and requires more sophisticated methods and equipment compared to an arterial blood gas analyzer. Finally, the COHb adjustments were not made for the ambient CO levels, potentially resulting in overestimating reported COHb levels. However, we believe that the controlled environment of the ICU setting mitigated these concerns.
Conclusion
In conclusion, we established the epidemiology of COHb level in first 24 hours and identified factors with a significant association with its level. Moreover, we identified that higher COHb levels were associated with longer ICU and hospital stay. Finally, we found that higher COHb levels were not associated with greater mortality. Taken together these observations suggest that COHb is unlikely to be a sufficiently robust biomarker for patient selection and risk stratification in future sepsis trials.
Conflicts of interest: The authors declared no potential conflicts of interest.
References
- Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014; 311(13): 1308-1316. doi:10.1001/jama.2014.2637.
- Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020; 46(8): 1552-1562. doi:10.1007/s00134-020-06151-x.
- Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med. 2009; 37(7): 2290-2298. doi:10.1097/CCM.0b013e3181a02afc.
- Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012; 25(4): 609-634. doi:10.1128/CMR.00016-12.
- Chung J, Chen C, Paw BH. Heme metabolism and erythropoiesis. Curr Opin Hematol. 2012; 19(3): 156-162. doi:10.1097/MOH.0b013e328351c48b.
- Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006; 86(2): 583-650. doi:10.1152/physrev.00011.2005.
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010; 50: 323-354. doi:10.1146/annurev.pharmtox.010909.105600.
- McArdle AJ, Webbe J, Sim K, et al. Determinants of Carboxyhemoglobin Levels and Relationship with Sepsis in a Retrospective Cohort of Preterm Neonates. PloS One. 2016; 11(8): e0161784. doi:10.1371/journal.pone.0161784.
- Kim TS, Choi DH. Liver Dysfunction in Sepsis. Korean J Gastroenterol Taehan Sohwagi Hakhoe Chi. 2020; 75(4): 182-187. doi:10.4166/kjg.2020.75.4.182
- Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010; 2(51): 51ra71. doi:10.1126/scitranslmed.3001118.
- Guney Varal I, Dogan P. Serial Carboxyhemoglobin Levels and Its Relationship with Late Onset Sepsis in Preterm Infants: An Observational Cohort Study. Fetal Pediatr Pathol. 2020; 39(2): 145-155. doi:10.1080/15513815.2019.1652377.
- Hara Y, Shinkai M, Kanoh S, et al. Arterial Carboxyhemoglobin Measurement Is Useful for Evaluating Pulmonary Inflammation in Subjects with Interstitial Lung Disease. Intern Med Tokyo Jpn. 2017; 56(6): 621-626. doi:10.2169/internalmedicine.56.7418.
- Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007; 4(10): e297. doi:10.1371/journal.pmed.0040297.
- Sebbane M, Claret PG, Mercier G, et al. Emergency department management of suspected carbon monoxide poisoning: role of pulse CO-oximetry. Respir Care. 2013; 58(10): 1614-1620. doi:10.4187/respcare.02313.
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997; 94(20): 10925-10930. doi:10.1073/pnas.94.20.10925.
- Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, et al. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am J Respir Cell Mol Biol. 1996; 14(6): 556-568. doi:10.1165/ajrcmb.14.6.8652184.
- Zegdi R, Perrin D, Burdin M, Boiteau R, Tenaillon A. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Med. 2002; 28(6): 793-796. doi:10.1007/s00134-002-1269-7.
- Shahin AA, Tamburro RF, Schmidt JE. Carboxyhemoglobin in pediatric sepsis and the systemic inflammatory response syndrome. Clin Intensive Care. 2000; 11(6): 311-317. doi:10.3109/tcic.11.6.311.317.
- Grigorescu BL, Săplăcan I, Bordea IR, et al. Endogenous Carboxyhemoglobin Level Variation in COVID-19 and Bacterial Sepsis: A Novel Approach? Microorganisms. 2022; 10(2). doi:10.3390/microorganisms10020305.
- Hampson NB. Carboxyhemoglobin: A primer for clinicians. Undersea Hyperb Med J Undersea Hyperb Med Soc Inc. 2018; 45(2): 165-171.
- Campbell NK, Fitzgerald HK, Dunne A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol. 2021; 21(7): 411-425. doi:10.1038/s41577-020-00491-x.
- Melley DD, Finney SJ, Elia A, Lagan AL, Quinlan GJ, et al. Arterial carboxyhemoglobin level and outcome in critically ill patients. Crit Care Med. 2007; 35(8): 1882-1887. doi:10.1097/01.CCM.0000275268.94404.43.
- Riquelme SA, Pogu J, Anegon I, Bueno SM, Kalergis AM. Carbon monoxide impairs mitochondria-dependent endosomal maturation and antigen presentation in dendritic cells. Eur J Immunol. 2015; 45(12): 3269-3288. doi:10.1002/eji.201545671.
- Maeda A, Pandey D, Inokuchi R, et al. Carboxyhemoglobin in Cardiac Surgery Patients and Its Association with Risk Factors and Biomarkers of Hemolysis. Anesth Analg. Published online. 2024. doi:10.1213/ANE.0000000000006915.
- Guillot JG, Weber JP, Savoie JY. Quantitative determination of carbon monoxide in blood by headspace gas chromatography. J Anal Toxicol. 1981; 5(6): 264-266. doi:10.1093/jat/5.6.264.
- Varlet V, De Croutte EL, Augsburger M, Mangin P. A new approach for the carbon monoxide (CO) exposure diagnosis: Measurement of total CO in human blood versus carboxyhemoglobin (HbCO). J Forensic Sci. 2013; 58(4): 1041-1046. doi:10.1111/1556-4029.12130.