SciBase Journals
SciBase Dentistry and Oral Sciences
ISSN 2996-363X
- Article Type: Review Article
- Volume 2, Issue 1
- Received: Apr 05, 2024
- Accepted: May 16, 2024
- Published Online: May 23, 2024
Polyamidoamine (PAMAM) Dendrimer induces Tooth Organizations Biomimetic Mineralization: A Review
Si Ning Li1,2; Le Qi1,2; Wan Tong Liu1,2; Zhiwei Li1,2; Yi Xin Fan1,2; Chen Kong1,2; He Zou1,2; Ji Wei Ren1,2; Zhi Hui Liu1,2*
1Hospital of Stomatology, Jilin University, 130021, People’s Republic of China.
2Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Changchun 130021, People’s Republic of China.
*Corresponding Author: Zhi Hui Liu
Hospital of Stomatology, Jilin University, 130021, People’s Republic of China.
Email: liu_zh@jlu.edu.cn
Abstract
The use of biomaterials to induce biomimetic mineralization of tooth tissue has always been a crucial issue in the treatment of dental caries. Polyamidoamine (PAMAM) dendrimer is a novel type of polymer nanomaterial with highly branched structure, definite shape, size, molecular weight, and excellent chemical and biological characteristics. It can be served as an organic template in biomimetic mineralization to induce and regulate mineral nucleation and crystal growth and has become a research hotspot in the field of biomimetic mineralization. This article provides a review of literature from 2000 to present day, aiming to summarize the recent role played by PAMAM in promoting biomimetic mineralization in hard tooth tissues.
Keywords: Polyamidoamine (PAMAM); Biomimetic mineralization; Hydroxyapatite (HA); Hard tooth tissue; Enamel; Dentine.
Citation: Li SN, Qi L, Liu WT, Li Z, Liu ZH, et al. Polyamidoamine (PAMAM) Dendrimer induces Tooth Organizations Biomimetic Mineralization: A Review. SciBase Dent Oral Sci. 2024; 2(1): 1012.
Introduction
Dental caries is a bacterial infectious disease and one of the most prevalent diseases in human beings. The World Health Organization has classified it as one of the three major prevention and treatment diseases, along with tumors and cardiovascular diseases [1]. Under normal circumstances, the teeth in the oral cavity maintain a dynamic balance between demineralization and remineralization. However, when the biofilm’s pH value remains low for an extended period due to acid production by bacteria in dental plaque biofilm, this equilibrium is to greater demineralization than remineralization and resulting in caries formation. Since the hard tooth tissue does not contain blood vessels and nerves, it cannot self-repair, research efforts focus on inhibiting early caries demineralization while promoting its remineralization [2]. At present, various fluoride products are commonly employed for remineralization treatment in clinics, such as fluoride gel, fluoride varnish, fluoride foam, etc.
Fluoride has a significant effect on preventing demineralization and facilitating remineralization, and its effectiveness against caries has been recognized. Nevertheless, the newly formed Hydroxyapatite (HA) usually lacks the structural and mechanical properties of natural enamel [3,4], and fluorine may potentially cause fluorosis and fluoride-resistant bacteria [5,6]. Furthermore, at present, the remineralization of most remineralizers does not involve spontaneous nucleation of mineral particles or precipitation of minerals in the matrix but rather the local growth of demineralized dentin residual nuclei in a calciumphosphorus ion environment [7-9]. Based on the nonclassical mineralization crystallization theory, scholars hope to address these problems from the perspective of biomimetic mineralization. Biomimetic mineralization refers to the incorporation of biomineralization mechanisms into the material preparation process. It involves simulating the microenvironment of the organism in vitro, utilizing the matrix material as a template, facilitating the formation of inorganic minerals on their surface through molecular biomimetic synthesis and molecular self-assembly techniques. This process allows for precise control over the composition and formation process of minerals, resulting in composite materials with exceptional biological and physicochemical properties similar to natural tooth tissue.
The main steps of biomimetic mineralization mainly involve (1) self-assembly of organic matrix macromolecules to provide nucleation templates for inorganic substances; (2) rapid deposition and recognition of inorganic-organic materials; (3) chemical vector regulation of crystal growth and assembly; (4) subunit mineral assembly leading to minerals with a natural biological structure [10-12]. The distinction between biomimetic mineralization and traditional remineralization lies in the fact that biomimetic remineralization triggers the formation of Amorphous Calcium Phosphate (ACP) through biomimetic molecules, replacing both free water and unbound water molecules within the collagen matrix to ultimately accomplish the mineralization of hard dental tissue. This process closely resembles natural biomimetic mineralization. Furthermore, similar to biomimetic mineralization, this mineralization process does not rely on preexisting apatite crystal cores within the collagen matrix. Biomimetic mineralization has great potential for repairing dental hard tissue defects. Materials with unique microstructure and desirable biological characteristics can be prepared by biomimetic mineralization. Research on biomimetic mineralization has mainly focuses on stabilizer functional energy analogs, template analogs such as Non-Collagenous Protein (NPCs) and amorphous nano-precursors.
Polyamidoamine (PAMAM) dendrimer have a large number of amide groups, similar to the links of peptide bonds in proteins, and their sizes match those of biological macromolecules, so they can be designed to resemble the structures of many proteins with different generations and structures and mimic the functions of proteins, and thus have been given the name of “artificial proteins”. In the process of biomineralization, organic macromolecules (proteins, polysaccharides) can be used as templates to nucleate and slowly grow inorganic materials at the inorganic-organic interface, and ultimately obtain composite materials with unique microstructures and biological characteristics. PAMAM provides a way to seek new biomimetic mineralized materials [13,14], which has become a hotspot of research on biomimetic mineralization. In this paper, we present a review of the research progress on the role of PAMAM in the biomimetic mineralization of dental hard tissues.
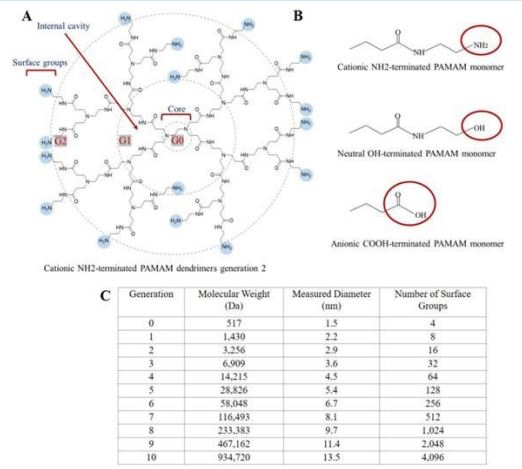
Polyamidoamine (PAMAM)
Polyamidoamine (PAMAM) (Figure 1) [15] is a polymer that was first synthesized by Tomalia et al [16]. Taking ammonia and ethylenediamine as the initiator core, with the polymerization reaction occurring gradually from the initiator core outward. The initiating nucleus undergoes a stepwise polymerization reaction outward, adding 1 “layer” or 1 “Generation” (G) to the formed polymer for each cycle, resulting in the formation of dendritic polymers with different number of generations, which are denoted as G0, G1, G2, G10 and so on. PAMAM is a three-dimensional structure consisting of three components: the initiator nucleus, an inner body composed of repeating units, and the outer surface of multiple tail functional groups [17]. PAMAM dendrimers have a highly regular structure, and their molecular weight, shape, and functional groups can be precisely controlled [18-21]. As generations increase, the molecular weight of PAMAM increases precisely while the surface group expands exponentially with each generation. Additionally, its radius increases approximately linearly at a rate of about 10 Å per generation. Consequently, at low generations, PAMAM exhibits a linear structure that transitions to a spherical shape when reaching four or more generations [20,22]. The high-generation PAMAM feature internal cavity, which can encapsulate organic or inorganic molecules such as metal complexes and nanoparticles [23,24], and the functional groups on the surface can be chemically modified and functionalized [20,25,26], thus enabling preparation of PAMAM dendrimers with diverse biological characteristics. The PAMAM dendrimer exhibits a threedimensional structure, highly branched architecture, internal cavities, and surface groups that can be easily modified. Additionally, it demonstrates excellent biocompatibility, low toxicity, and no immunogenicity, making it the most extensively investigated class of dendrimers [27,28]. PAMAM dendrimers can affect the formation and morphology of Hydroxyapatite (HA) crystals and can be combined on the surface of demineralized collagen fibers as a template for crystal nucleation to induce HA crystals with similar structure and orientation to natural enamel dentin crystals. Therefore, PAMAM dendrimers are considered biomimetic mineralized molecule.
Interaction between PAMAM and HA
Recently PAMAM dendrimers have been extensively studied in the crystallization process of HA. PAMAM polymers with different generations, surface groups, and concentrations can regulate the size and shape of HA. Naka et al reported that the presence or absence of PAMAM can adjust the morphology of CaCO3 crystals to form spherical vararite or rhombohedral calcite respectively [29]. In addition, it was found that without any PAMAM or using a lower generation such as G1.5, it is not possible to form composite films with CaCO3 /poly (ethyleneimine) [30]. Zhang et al discovered that the choice of generation and concentration of PAMAM dendrimers with carboxylic acid groups on the outer surface has great influence on the morphology and size of BaWO4 crystals. As the generation number of PAMAM dendrimers increased from G1.5 to G3.5, the diameter of the crystals increased from 0.6 to 5 mm, promoting the formation of crystals, while dendrimers with higher generation acted as an inhibitor of crystal formation [31]. In addition, the crystal size change of G4-PAMAM synthesized by the hydrothermal method with amide groups on the outer surface is dependent on PAMAM generation. The shape and dimensions of the crystals were also related to the concentration of PAMAM dendrimers [32]. The mineralization of Crystal occurrence is initiated through calcium complexation by the concerted action of NH2 and amide C=O groups [33]. Further studies revealed that powdery nanorod crystals with narrow particle size distribution were prepared hydrothermally under G5-PAMAM-COOH, which also confirmed that PAMAM dendrimers appeared to be inhibitors of crystal initiation and influenced the crystal morphology and size during the in vitro mineralization [34]. Khopade and his colleagues utilized carboxylic acid-terminated half-generation PAMAM dendrimer as a template to prepare amorphous spherical HA. They suggested that the PAMAM dendrimers may act as nucleation sites due to calcium binding to the carboxylic acid groups present on their surface [35]. Furthermore, PAMAM dendrimers with carboxyl groups and polyhydroxy groups had an impact on the size and shape of the HA. The internal nitrogen portion of the PAMAM dendrimers serves as a complexation site, the core is hydrophilic and may be open to calcium ions, especially in the case of polyhydroxy-terminated PAMAM, so it is hypothesized that the nucleation sites of PAMAM are located either outside or inside of PAMAM [36,37]. Xie et al investigated the effect of G2-PAMAM, synthesized using glutamic acid (MG) as a shell on the crystallization of calcium phosphate. In the presence of PAMAM-MG, the morphology of calcium phosphate crystals exhibited a band-like structure with reduced thickness and width compared to dendrimer-free crystals. This outcome can be attributed to charge interactions between the dendrimer and octacalcium phosphate. Additionally, PAMAMMG demonstrated an affinity for gelatin and potentially contributed to the formation of Amorphous Calcium Phosphate (ACP). These findings indicted that PAMAM-MG had potential as a modulator for controlling OCP morphology [38]. The above results indicate that the number of generations of PAMAM, the terminal groups, the concentration, the nucleation site, and the relative growth rate of the crystallization surface can all affect the morphology and size of HA. Different generations and different terminal groups of PAMAM have distinct binding sites with metal ions, these ions can be either bound to the surface of PAMAM or to the nitrogen-containing parts inside PAMAM, which leads to the adsorption of different PAMAMs to different growth planes and changes the relative growth rates of different crystal planes, and thus regulates the formation of diverse crystal morphologies. The high generation of PAMAM (3.5) has an inhibitory effect on crystal growth due to its spherical shape with limited space within its internal nitrogen-containing region.
Effect of PAMAM on remineralization of enamel
Biomineralization of tooth enamel
Enamel consists of phosphate crystals secreted by ameloblasts from the inside out. Mature enamel is a highly mineralized acellular tissue. When damaged by external or endogenous factors, there are no effective physiological mechanisms to repair except for the protection and remineralization potential provided by saliva. Enamel biomineralization is a process that involves the coordination of organic substrates and inorganic crystals, which are regulated by genetic programs within cells. The formation of enamel starts from the enamel-dentin junction, which can be divided into three stages: secretion, transition, and maturity [39]. The main mineral secreted during the early stages of enamel formation is ACP, the mineralization precursor, which is regulated by enamel matrix proteins to assemble into highly ordered and arranged HA [40]. Enamel matrix proteins are the main regulators of biomineralization and are important for the initiation, orientation, and nucleation growth of crystals, and mainly include amelogenin protein and non-amelogenin protein (e.g., ameloblastin, enamelin and tuftelin) [41]. Amelogenin constitutes 90% of the enamel matrix and plays a pivotal role in enamel formation. The N-terminal region of amelogenin interacts with calcium and phosphorus ions at the stage of enamel formation, and the C-terminal end orchestrates HA to be highly ordered [42-44]. The hydrophobic N-terminal and hydrophilic C-terminal jointly endow the protein with amphiphilicity, which enable self-assembly into nanospheres, chains, and ribbons to stabilize ACP, provide a structural template for ACP growth, and induce and regulate the growth of enamel crystals [43,45-47]. The electrostatic adsorption between the functional end groupof amelogenin and the binding site of HA crystals is a critical step in the remineralization of HAcrystals guided by amelogenin [48]. Therefore, the primary objective of enamel biomimetic mineralization is to replicate the characteristics of HA assembly and regulate HA crystal nucleation, orientation, formation, and growth through the self-assembly of amelogenin or their mimetics as an organic template for mineralization.
PAMAM induces biomimetic mineralization of enamel
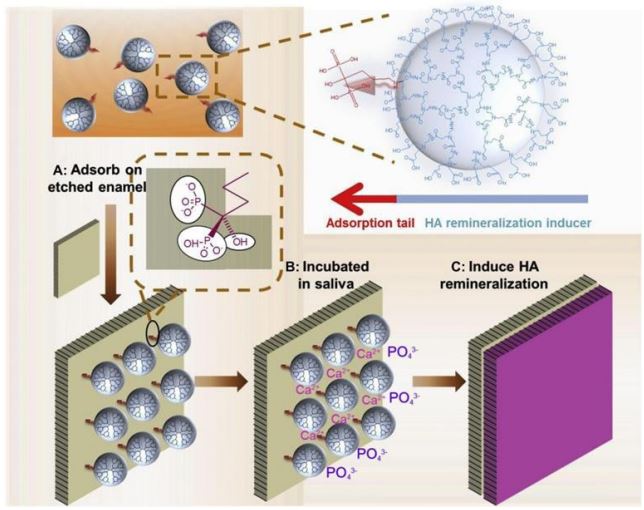
PAMAM exhibits a strong tendency to self-assemble into hierarchical enamel crystal structures. In an aqueous solution, G3.5-PAMAM-COOH and G4-PAMAM-NH2 formed fractal aggregates by electrostatic interaction. The studies of aggregation dynamics indicated that diffusion controlled colloidal aggregation (DLCA) dominated the electrostatic assembly of higher generation PAMAM dendrimers. Under suitable conditions, electrostatic self-assembly between PAMAM-COOH and PAMAM-NH2 can serve as an efficient synthesis method for generating higher-order complex architectures such as fractals [49]. PAMAMCOOH self-assembled in aqueous solutions containing ferric chloride of different pH values to form nanorods, nano strands, microspheres of similar morphologies, and finally formed stable microstrip aggregates with a length of millimeters, width of micrometers, and thickness of nearly 5 mm the change of pH value did not affect the morphology of PAMAM aggregates. The mechanism of microribbon formation remains is still unknown. It was proposed that the carboxylic groups on the surface of the PAMAM-COOH dendrimer provide the complexation sites with the Fe3+ ion. When the PAMAM-COOH dendrimer was added to ferric chloride aqueous solution, the Fe3+ cations may be adsorbed on the peripheral carboxylate radical anions through the electrostatic interaction. This would rapidly form a positively charged PAMAM and bind to the negatively charged carboxyl groups of the remaining PAMAM in the aqueous solution. In such a case, these Fe3+ cations act as a linker between dendrimers promoting the self-assembly process. In addition, once occupied by Fe3+ cations, no free COOH groups would be present at the surface, making these occupied parts impossible for the association with the positively charged dendrimers. As a result, only several external groups of the PAMAM monomer had the opportunity to be connected with each other, which may explain the directional aggregation of PAMAM into a fiber-like structure rather than bigger spheres in solution. PAMAM may be an ideal building block to mimic the self-assembly of amelogenin [50]. Yang et al. reported a novel amphiphilic PAMAM dendrimer, whose periphery was modified with l-aspartic acid and an aliphatic chain at the focal point (Sa-PAMAM-Asp), in or-der to simulate the self-assembly behavior, the function of amelogenin, and the inter-actions between amelogenin’s Cterminal aspartic acid residues and HA, to establish an optional environment for the growth of HA in vitro. Sa-PAMAM-Asp was initially self-assembled into nanospheres and subsequently converted into linear chains through increased concentration or additive incorporation. The linear component demonstrates a functionality akin to that of amelogenin in guiding HA growth. The c-axis of the HA crystals is preferentially oriented along the amelogenin aggregates, similar to that characterized by the lowest level of enamel-layered structures [51]. This study has provided direct experimental evidence for protein-mediated nucleation and growth of HA as well as reveals the mechanism of enamel biomineralization. The phosphorylated PAMAM with peripheral groups of triphosphate or bisphosphonate designed by Xin et al. can serve as a mineralization template for mineralization to generate fishbone-like or fibrous HA nanocrystals. The micro-scale phosphorylated PAMAM were self-assembled into ribbons or protofibrils that could induce HA deposition and generate mineralized networks [52]. The above studies supply a theoretical foundation for the PAMAM self-assembly process and support its application in dental enamel biomimetic mineralization. PAMAM acts as an organic template on the surface of demineralized enamel, inducing the formation of HA, which has the same structure, orientation, and minerals as natural enamel. Chen et al immersed demineralized enamel in 1000 ppm PAMAM-COOH solution for 30 minutes and then in fluorinated and non-fluorinated calcium phosphate solutions for 20 hours. It was found that PAMAM-COOH could be used as a template for mineralization to induce the formation of HA crystals with the same structure, orientation, and mineral as intact enamel in a relatively short period [53]. The carboxyl groups bound to calcium ions on the surface of HA via coordination bonds, providing a firm combination for PAMAM and enamel. In this study due to demineralization, the peripheral region of the enamel prism was more severely decalcified than the central region, resulting in more c-axis surfaces of the enamel crystals being exposed, which could adsorb more PAMAM self-assemblies, and PAMAM-COOH preferentially adsorbed with the c-axis on the crystal surfaces in order to increase the nucleation and growth of new crystals around the enamel prism. In addition, PAMAM-COOH assemblies can act as a linker between the new crystals on the enamel surface and the new crystals in solution, thus determining the arrangement of the regenerated crystals. PAMAM-COOH attracts and stabilizes ACP in solution, and it funcytioned as a template to to organize these nanoprecursors and guide their crystallization along the “amorphous precursor pathway [54].
The previous studies have used strong acids to demineralize the superficial layer of enamel to create enamel caries, a condition that creates a highly calcified zone of approximately 30 mm in the outermost enamel layer [55]. This results in the outer enamel layer being highly resistant to acid compared to the inner enamel layer. To better simulate the physiological environment and acid challenge during meals in the oral cavity, Fan et al. developed a subsurface demineralization model and implemented a demineralization-remineralization cycle to more accurately predict the actual effects of PAMAM-induced mineralization. For the first time, they quantified the mineralization capacity of PAMAM with different terminal groups. It was shown that PAMAM-COOH, PAMAM-OH, and PAMAM-NH2 all had the ability to induce enamel remineralization. The percentages of remineralization for the PAMAM-NH2, PAMAM-COOH, and PAMAM-OH groups were 76.42±3.32%, 60.07±5.92%, and 54.52±7.81%, respectively [56]. The positively charged PAMAMNH2 binds to the negatively charged enamel surface through electrostatic forces involving positive and negative charges [57]. PAMAM-NH2 exhibited superior performance by significantly enhancing hardness recovery in the cross section while minimizing damage depth and mineral loss. On the other hand, PAMAM-COOH forms a coordination bond with calcium ion on the HA surface via its carboxyl group [37], thereby inducing subsurface enamel mineralization. However, PAMAM-OH, which is electrically neutral [58], does not effectively repair demineralized enamel. Jia et al also showed favorable remineralization ability of PAMAM-COOH and PAMAM-NH2 on the enamel surface. The regenerated crystals exhibited ca/p ratios of 1.663 and 1.637, which closely resembled that of HA (1.67) in the original enamel, indicating successful induced remineralization with consequential changes in the three-dimensional morphology of the enamel surface. Notably, there was a significant reduction in the height of enamel rods, resulting in a smoother sur-face that hindered Streptococcus mutans adhesion. Although there was no significant difference in surface roughness between these two groups, the PAMAM-COOH group exhibited a higher bacterial adhesion force compared to the PAMAM-NH2 group [59]. Surfaces with similar roughness may possess distinct geometric details, symmetries, or hierarchical arrangements of surface features [60], as well as the inhibition of positively charged PAMAM-NH2 on bacteria [61], which can all influence bacterial adhesion. Consequently, the enamel induced by PAMAM-COOH and PAMAM-NH2 demonstrates resistance against oral bacteria and reduces secondary caries formation, making it a promising material for early enamel caries treatment.
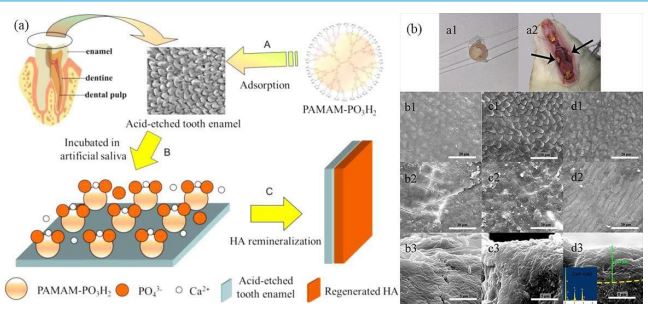
The application of PAMAM as a remineralization template is limited by its weak binding ability with HA, and it is easily detaching from the enamel surface in the daily complex oral environment, inhibiting the mineralization process of mineral crystals on specific surfaces, which is similar to the inhibition of CaCO3 formation by free dendrimer in aqueous solution [62]. Therefore, it is crucial to improve the binding capacity between PAMAM and human hard tissue matrix, particularly HA, for achieving in situ regeneration or remineralization. Chen et al used PAMAM with different terminal groups as probes to investigate the charge distribution on the HA surface in rat enamel. They discovered that PAMAM with carboxyl, amino, and acetylamino groups could be attached to the enamel surface through charge adsorption or complexation mechanisms and that the binding strength of PAMAM to the crystals depended on the terminal groups of PAMAM, the order of the binding strength of the two was positively charged amino group > negatively charged carboxyl group > neutral acetylamino group, and meanwhile an alternating positive and negative charge band was found on the surface of the crystals [63]. This result complements that of Kirkham et al, who showedthat the alternating domains of surface charge consisted of broad bands approximately 30 to 50 nm wide using chemical force microscopy [64]. Furthermore, G7-PAMAM-COOH was employed as a probe for hydroxyapatite nanorods to establish a mathematical modeling for understanding the binding capacity between crystal surfaces and PAMA molecules. The study indicated that the dendrimers were separated along the crystal C-axis with a binding strength measuring 90±20 kJ/mol [65]. These above findings suggest that the binding strength between PAMAM and HA crystals depends not only on the terminal groups of PAMAM, but also on the charge strengths of the protein and crystal surfaces. Modulating the terminal group of PAMAM can initiate or regulate the growth of HA crystals, leading to varying degrees of binding affinity with the crystal surface. Wu et al designed a composite material consisting of Alendronate sodium (ALN) anchored to HA and PAMAM-COOH, which utilized the specific adsorption of ALN on enamel to enable ALN-PAMAM-COOH to be firmly bonded to the enamel and be able to resist Phosphate Buffered Saline (PBS) rinsing to fully exert the PAMAM-COOH induced in situ remineralization of HA, and the regenerated HA had a nanorod-like crystal structure similar to that of human enamel, with the thickness of the newly generated mineralized layer reaching 11.4 μm, and restored the microhardness of acid-etched enamel samples to 95.5% of the original value, with a Ca/P ratio of 1.67, which was very close to that of the original value of natural enamel. Animal experiments also demonstrated that ALN-PAMAM-COOH could effectively induce the mineralization of HA in rat oral cavities (Figure 2) [66]. However, the complex synthesis process involved in producing ALN-PAMAM-COOH may impede its further industrialization and potential biomedical applications. Inspired by the structure and properties of amelogenin, Chen et al prepared PAMAM-PO3H2 , One reason is that the phosphate group exhibits a higher affinity for calcium ions compared to the carboxyl group, while another reason lies in its strong binding ability with HA, which can simultaneously fulfill the functions of both ALN and PAMAM-COOH parts in ALN-PAMAM-COOH, and PAMAM-PO3H2 can simulate the natural process of amelogenin regulating biomineralization during enamel development, inducing the production of HA crystals that are highly ordered and predominantly oriented along the Z-axis of the pristine enamel surface, with low cytotoxicity.
Animal experiments have also confirmed that PAMAM-PO3H2 can effectively induce significant regeneration of enamel (Figure 3) [67]. Gao et al. combined the nucleating template PAMAMNH2 with Amorphous Calcium Phosphate (ACP) to enhance the enamel remineralization, and utilized the adsorption function of statherin on HA to improve the binding capacity of PAMAMNH2 on HA, to prepare a DpSpSEEKFLRRIGRFG-functionalized PAMAM-NH2 (called SN15-PAMAM), ACP releases high levels of calciumphosphorus ions, which rapidly neutralize acid to protect the tooth, and calciumphosphorus ions in the solution stabilize the structure of SN15, which leads to a stronger bonding of PAMAM-NH2 to enamel and promotes remineralization. Compared with the control group, the SN15-PAMAM+ACP adhesion method achieved a 90% increase in enamel remineralization for artificial caries [68]. Therefore, the SN15-PAMAM+ACP adhesive method reduces the remineralization of enamel damage, increases the hardness, improves the service life of the interface of the restored teeth, and reduces secondary caries. Tao et al. constructed a honokiol-loaded PAMAM dendrimer (PAMH) by a cosolvation method to counteract early enamel caries. PAMH induced enamel remineralization and sustained the release of antimicrobial drugs at the same time, which resulted in long-term antimicrobial activity. The release of honokiol in the cariogenic low pH environment is slower and more sustained than that in a neutral environment due to electrostatic interaction, where P3 beads with the same charge repel each other, leading to the rapid expansion of the PAMAM molecules and accelerating the release of the drug. However, protonated P1 beads have a positive charge while protonated P3 beads have no charge at low pH; thus, electrostatic repulsive interactions between protonated P1 beads are limited by the outermost layer of P3 beads on the surface of the dendrimer, resulting in slow swelling rate and drug release under acidic conditions. Animal experiments also shown that PAMH has a long-term effect on rat dental caries and exhibited good biocompatible [69]. Therefore, PAMH has a broad application prospect in enamel restoration.
Effect of PAMAM on remineralization of dentin
Biomineralization of tooth dentin
The formation of dentin, similar to dental enamel, is regulated by odontoblasts and the extracellular matrix. Dentin biomineralization involves the secretion of an organic matrix and the deposition of crystals. Under physiological conditions, odontoblasts secrete an extracellular matrix primarily composed of type I collagen, accounting for 90% of its composition. This collagen serves as a scaffold for mineral crystal deposition [70]. Non-Collagenous Proteins (NCPs) accounted for 10%. The structure of NCPs is rich in aspartic acid, glutamic acid, serine and other acidic amino acid residues, with a large number of negative charges, and has a strong calcium ion and HA binding ability [71], which reduces the nucleation activation energy and driving force required for apatite formation [72], and acts as the initiating factor, stabilizing factor or inhibiting factor of mineralization to regulate mineral deposition. NCPs can induce the formation of stable ACP in the presence of calcium and phosphorus ions. The smaller nano precursors can freely penetrate into the demineralized dentin collagen fibre matrix and align along the microfibers within the collagen fibres, forming nanomicrospheres with potential to transition into apatite nanocrystals. Simultaneously, NCPs can also bind to specific regions of collagen fibres (such as vacant areas), attracting a significant number of nanocrystals into the demineralized dentin matrix by providing a template based on matrix phosphorylated proteins. This process allows for control over both size and level of HA facilitating complete intra-fibrous mineralization of dentin [73,74]. The collagen framework plays a crucial role in facilitating dentin remineralization in a three-dimensional manner. Recent evidence has demonstrated that collagen fibres possess the ability to regulate the size and shape of apatite [75], while the charged groups within collagen serve as nucleation sites for promoting apatite formation. However, it should be noted that the rate of nucleation is relatively slow [76]. Therefore, the collagen acts as a scaffold, while the NCPs serve as a mineralization template, working together to facilitate the orderly growth and deposition of crystals, ultimately leading to the formation of mineralized dentin [72].
The NCPs encompass Dentin Matrix Protein 1 (DMP 1), Dentin Phosphoproteins (DPP), and Dentin Salivatin (DSP) [77]. DMP 1 is a tissue-specific NCP involved in mineralization within the dentin matrix, cleaving two dysfunctional fragments in the cytoplasmic matrix. The C-terminal segment, abundant in carboxyl groups, facilitates the phase transition of ACP to HA, thereby inducing extrafibrillar mineralization of collagen fibrils. The N-terminal fragment, rich in aspartic acid residues, aids in stabilizing amorphous calcium phosphate into nanoparticles and subsequently induces intrafibrillar mineralization of collagen fibrils [74,78]. DMP1 is capable of stabilizing calcium and phosphorus ions in solution to form nucleating precursor [79,80]. Through electrostatic adsorption, these nucleating precursors aggregate within dentin collagen fibres to generate ACP nanoparticles, which subsequently undergo crystal orientation to develop single apatite grains within the collagen molecular gap [81]. In conclusion, under physiological conditions, DMP1 possesses the ability to bind collagen fibres and calcium ions while inducing the formation of HA, an essential NCP involved in regulating dentin mineralization [82]. However, in mature dentin, the remineralization ability of NCPs diminishes, making it challenging to extract natural NCPs. Therefore, the concept of dentin biomimetic mineralization aims to identify NCPs analogues that can stabilize mineralized precursors and serve as nucleation templates. PAMAM dendrimers belong to a class of monodisperse nanomaterials that exhibit similar chelating and templating effects as NCPs. When present in solution, PAMAM dendrimers can impede mineralization and stabilize ACP nano precursors to prevent phase transition. Moreover, when bound to the surface, PAMAM dendrimers can act as nucleation templates for inducing remineralization.
PAMAM induces biomimetic mineralization of dentin
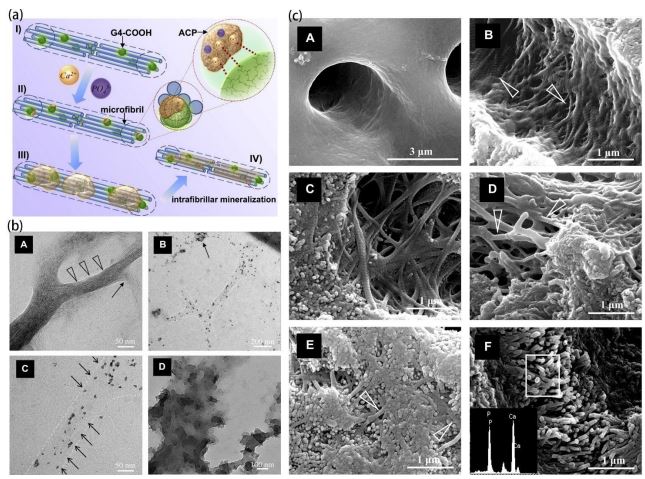
The biomimetic mineralization of dentin, in contrast to enamel, involves both intra-fiber and inter-fiber mineralization. Inter-fiber mineralization alone, which is deposited only on the dentin surface, cannot generate a highly mineralized collagen matrix [83]. Optimal mineralization can only be achieved when both inter-fiber and intra-fiber mineralization occur simultaneously. Therefore, the challenge in achieving biomimetic dentin mineralization lies in the ability of ACP to penetrate collagen fibers and nucleate and mineralize at specific sites. Li et al used the monodispersity of G4-PAMAM-COOH and the size scale topology similar to protein to simulate the function of NCPs, which was inspired by the natural mineralization process guided by NCPs. PAMAM-COOH combines with collagen fibrils at specific sites and remains in collagen fibers through size exclusion. The carboxyl groups in PAMAM-COOH attract and stabilize ACP nano precursors in solution, aggregating them into the collagen matrix, Additionally, the internal nitrogen portion and intramolecular space of PAMAM-COOH can serve as complexation sites for nano-precursors [62]. Consequently, the dendritic polymer can act as a template to order the nano precursors and guide these nanocrystals to crystallize along the “amorphous precursor pathway”, thus achieving intrafibrillar mineralization along the microfibrils in the collagen matrix (Figure 4) [84]. PAMAMCOOH can play the dual functions of natural NCPs in this process, that is, mineral ion sequestration and mineralization template. As such, it can achieve intrafibrillar mineralization with a hierarchical structure both in vitro and in vivo. Xie et al. sutured a PAMAM-COOH-treated model of demineralized dentin onto rat buccal mucosa to replicate the authentic environment of the human oral cavity and the cavity. The results showed that PAMAM-COOH effectively promoted the biomineralization of demineralized dentin in vivo, and also exhibited a favorable performance in the blockage of dentin tubules [85]. Similarly, G4- PAMAM-PO3H2 is similar to DPP in size, topological structure, and peripheral function, and is anchored in collagen matrix with excellent dentin binding ability by size exclusion and electrostatic interaction with cross-linked collagen [86], and plays the role of dentin DPP analog in vivo and in vitro experiments. The dentin DPP analogue, PAMAM-PO3H2 , effectively induced remineralization of the regenerated dentin mineral layer both in vivo and in vitro, with a thickness exceeding 10 um [87]. Another showed that PAMAM-PO3H2 at concentrations less than 100 mg /mL were promoters for apatite nucleation growth but inhibitors at concentrations greater than 500 mg /mL. PAMAM-PO3H2 also mimicked biomineralization through the stabilization of ACP and were multifunctional NCPs analog for biomimetic remineralization [88]. Liang et al. revealed that PAMAM-NH2 , with its highly ordered structure and numerous calcium coordination sites, has the good binding ability to demineralized dentin, induces needle-like crystals to precipitate on the dentin surface and in dentin tubules, and regenerates minerals with an excellent acid resistance [89]. Most notably, PAMAM-NH2 is able to facilitate the mineralization of type I collagen fibers to achieve collagen fiber of endo mineralization. Therefore, PAMAM-NH2 may be an ideal template for inducing dentin remineralization in completely demineralized collagen mineralized tissue. Nevertheless, compared to other types of PAMAN dendrimers, PAMANH2 exhibits slightly inferior biocompatibility [90]. The results of the quantitative study on the mineralization ability of PAMAM in dentin showed that both PAMAM-NH2 and PA-MAM-COOH induced dentinal tubules with reduced mineral loss, shallower damage depth, and higher blocking rate compared to PAMAMOH. There were no significant differences observed between PAMAM-NH2 and PAMAM-COOH in terms of mineral loss, depth of dentin damage, hardness, rate of dentin tubule obstruction, as well as the percentage of calcium and phosphorus elements. There were no significant differences between PAMAM-NH2 and PAMAM-COOH in terms of the depth of dentin damage, dentin hardness, dentin tubule blockage rate, and the percentage of calcium and phosphorus. The remineralization rates for PAMAM-NH2, PAMAM-COOH, and PAMAM-OH were 82.18± 2.96%, 76.34±4.53%, and 56.30±8.31% respectively [91]. The differences were attributed to the variations in charge of the terminal group among these three different types of PAMAM molecules as well as their differential electrostatic binding abilities towards collagen fibers. Furthermore, carboxyl > amino > OH groups exhibit varying degrees of affinity towards functional groups and calcium ions.
PAMAM-COOH and PAMAM- PO3H2 both guide collagen protofibrils towards intrafibrillar remineralization. However, PAMAM-COOH or PAMAM-PO3H2 cannot fully recapitulate the mechanical properties exhibited by naturally mineralized tissues at the nanoscale level [66]. Qin used PAMAM-PO3H2 to replicate the isolated functional motifs of the N-terminal fragment of DMP1, and PAMAM-COOH to mimic the template functional motifs of the C-terminal fragment of DMP1. The combined application of PAMAM-PO3H2 and PAMAM-COOH successfully induced dentin intra-fiber mineralization. Notably, PAMAM-PO3H2 has good binding strength to the surface of demineralized dentin, and during the amorphous precursor induction phase, the suitable ACP were able to penetrate into demineralized collagen matrix with assistance from PAMAMCOOH. Furthermore, HA nucleation and growth were effectively induced by PAMAM-PO3H2 . Both dendrimers showed promising results in mineral regeneration as they partially covered dentin surface and tubules. However, it should be noted that control groups treated with Sodium Tripolyphosphate (STPP) and Poly Acrylic Acid (PAA) immersion displayed higher levels of mineralization within demineralized collagen fibrils and filled dentinal tubules. The ratios of remineralization for the two methods were 1.11 and 1.25 respectively, and the control group was closer to healthy dentin [88]. Nevertheless, it is worth mentioning that control group results resembled those observed in healthy dentin more closely than those achieved by using either PAMAM-PO3 H2 or PAMAM-COOH alone. On the one hand, although the modified dendrimer PAMAM has the ability to bind a significant number of functional groups, it may not possess as many functional groups as the STPP and PAA groups in the control group. Another reason could be that the induction concentrations of 2.5% STPP and 1000 ug/mL PAA applied for remineralization in the control group are considered optimal, while the experimental group concentration aligns with that of the control group; however, this concentration might not be ideal for achieving biomimetic remineralization using PAMAMPO3H2 and PAMAM-COOH. Therefore, further experiments are required to investigate the optimal concentration of PAMAMPO3H2 and PAMAM-COOH for biomimetic remineralization. However, it is undeniable that phosphorylated PAMAM and carboxylated PAMAM dendrimers hold great potential in the clinical treatment of dentin demineralization. On this basis, Xie et al. explored the mineralization mechanism of PAMAM-PO3H2 and PAMAM-COOH on type I collagen fibrils and constructed a twodimensional collagen fiber model of recombinant type I collagen fibrils that is easy to observe. The mineral formed in the PAMAM-PO3H2 and STPP groups was predominantly polycrystalline phase minerals, whereas no crystals were detected in either the PAA or PAMAM-COOH groups. This indicated that the phosphate groups may play a role in promoting nucleation. The change in fiber diameter was relatively insignificant in the STPP + PAA group, whereas it was highly significant in the PAMAMCOOH and PAA groups. Therefore, it can be inferred that the presence of carboxyl groups plays a crucial role in the process of crystal maturation. The combination of PAMA-MCOOH and PAMAM-PO3H2 could induce natural hydroxyapatite crystals with enhanced maturity that initiated mineralization within 24 hours, resulting in an overall faster mineralization process. High concentrations of phosphate groups may hinder crystal maturation; conversely, high concentrations of carboxyl groups can promote it. However, the synergistic effect between these two factors to achieve optimal remineralization is not further discussed in this studied.
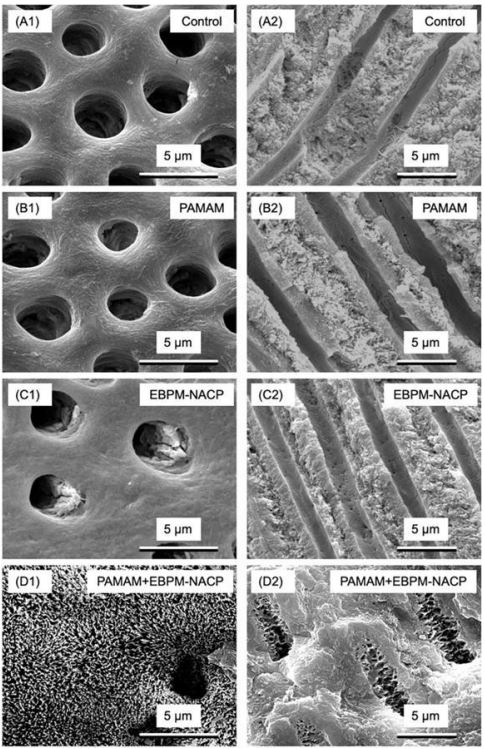
PAMAM is an excellent nucleation template, which can quickly absorb calcium and phosphorus ions to remineralization. However, PAMAM itself cannot release calcium and phosphorus ions, neutralize acid, and raise pH value to resist demineralization. Yang et al. synthesized PAMAM-COOH/NACP composites, which effectively transports NACP into the intrafibrillar space of the protofibrils to induce mineralization within the fibril structure, and innovatively used fluorescent labeling technology for observation and evaluation of the remineralization effect. PAMAM-COOH/NACP showed remarkable intrafibrillar mineralization of human dentin type I collagen fibers and facilitated mineral maturation, with the PAMAM-COOH/NACP group forming a mineral Ca/P ratio of 1.9, which closely resembled that of untreated dentin [92]. Previous research on tooth remineralization was mainly performed in saliva-like solutions, which contain calcium and phosphorus ions and pH of 7 to simulate the remineralization function of human saliva. However, when tested in a circulating artificial saliva/lactic acid model that more closely matches the actual oral environment, it was observed that the biomimetic mineralization effect of PAMAM was significantly weaker compared to previous studies conducted at a constant pH of 7 [89]. Liang et al. investigated the effects of PAMAM + NACP bonding agent on dentin remineralization in a cyclic artificial saliva/acid challenge environment for the first time. PAMAM+NACP composite offer triple advantages, including serving as a nucleation template, neutralizing acid, and releasing calcium and phosphorus ions. During the challenge of the artificial saliva lactic acid cycle (soaking in artificial saliva at pH 7 for 1 hour, soaking in artificial saliva with pH 4 for 1 hour for 21 days), this resulted in a significant increase in hardness, approaching that of healthy dentin, indicating complete remineralization of demineralized dentin. It was proved complete remineralization of demineralized dentin. In addition, prior to bonding, dentin was treated with PAMAM at a concentration of 10 mg/ml. The adhesive was supplemented with NACP and the composite was bonded using a NACP Interestingly, varying the NACP content in the adhesive from 0% to 40% had no impact on the bond strength. Remarkably, employing PAMAM+NACP bonding technique successfully restored the hardness of demineralized dentin to that of normal dentin even when subjected to artificial saliva lactate cycling challenge [93,94]. This novel approach holds great promise in enhancing the durability of resin dentin bonds, preventing dental caries However, it is important to note that although the cyclic artificial saliva/lactate protocol simulates common oral conditions, it does not fully represent an oral environment characterized by dry mouth and localized low pH. Liang et al contacted PAMAM impregnated dentin samples with 40% NACP adhesive in a lactic acid solution at a pH value of 4 for 24 hours over a period of 14 days to simulate the most challenging oral environment of patients with severe saliva reduction or deficiency. The results showed that PAMAM + NACP resulted in complete remineralization of demineralized dentin with increased hardness close to healthy dentin at pH4 conditions without the presence of calcium and phosphorus ions, and the new PAMAM + NACP method is expected to increase the longevity of composite dental bonding, prevent caries, mineralize lesions and protect tooth structure, even in patients with dry mouth and acidic oral environments [95,96]. Although PAMAM+NACP demonstrated superior remineralization, the ability of NACP to release calcium and phosphate ions and neutralize acids decreased over time. In addition, the fluid flow in the oral cavity washes away PAMAM and loses its role as a nucleation template, so it is necessary to develop methods to maintain long-term mineralization despite the existence of fluid impact. Liang et al subjected PAMAM-coated demineralized dentin samples to 72/77 days of PBS immersion and shock to construct an accelerated fluid attack model to simulate 1 year of fluid stimulation in the oral cavity. Then, the rechargeable NACP (EBPM-NACP) was immersed in lactic acid at pH 4 for 72/77 days to completely empty calcium and phosphorus ions. The exhausted EBPM-NACP was recharged with a solution of calcium and phosphorus ions using a simulated mouthwash before being exposed to PAMAM-soaked dentin samples and challenged with artificial saliva containing lactic acid for 35 days. Following 72 days of fluid oscillation, a significant portion of PAMAM remained attached to the dentin surface, retaining its function as a nucleation template. It is worth noting that the acid neutralization and ion re-release capabilities of the PAMAM+EBPMNACP composite did not decrease even after repeated charging and re-release cycles, which indicated the long-term remineralization energy and the mineralization of samples using this strategy of PAM AM+EBPM-NACP almost recovered to the level of healthy dentin (Figure 5) [97-99]. The triple benefit achieved by submerged PAMAM + EBPM-NACP composites compared to PAMAM + NACP is the provision of superior nucleation templates, sustained charge re-release of calcium and phosphorus ions, and acid neutralization even under prolonged fluid turbulence conditions. The novel PAMAM +EBPM-NACP composite approach is expected to provide long-term dental protection and caries prevention, particularly for individuals with dry mouth, reduced saliva flow, and radiographic dental caries.
Dentin Hypersensitivity (DH) is the most common symptom. The occlusion of Dentinal Tubules (DTs), both in the short-term and long-term, plays a crucial role in treating DH. Short-term closure quickly relieves symptoms, while long-term occlusion is related to the intra-fibrous mineralization of DTs and can resist the re-exposure of dentinal tubules caused by acids in food. PAMAM-OH has a good binding capacity with demineralized dentin and induces effective dentin tubule occlusion against acid attack after 7 days [100]. Wen et al used PAMAM- PO3H2 to induce the remineralization of DTs and sealing of DTs. Although deep remineralization was not achieved with PAMAM-PO3H2 , it successfully sealed DTs through complete mineralization. Moreover PAMAM-PO3H2 succeeded in inducing HA in demineralized dentin and reconstructed type I collagen matrix, suggesting PAMAM-PO3H2 may be a future clinical biomaterial for sealing DTs [101]. However, one limitation of this experiment is that the existence of a large number of type I collagen matrix in DTs may affect the nucleation and growth of crystals [102]. Gao et al. evaluated the stability of dentin tubule occlusion by immersing demineralized dentin samples coated with NaF and PAMAMNH2 in artificial saliva after brushing and acid challenge respectively. After 14 days of immersion in artificial saliva, the mineralization induced by NaF was significantly faster than that induced by PAMAM, because the mineralization precipitation resulting from the inorganic reaction with NaF occurred at a higher rate than the biomimetic mineralization induced by PAMAM. 28 days later, the mineralization rates of PAMAM were similar to that of the NAF group, but only a few millimeters of mineralization were deposited in the dentinal tubules of the NaF group, while the dentinal tubules of the PAMAM group were filled with needle-like crystals. The deposition rate of NaF was very fast, with the surface of the dentinal tubules quickly closed, so it was difficult for Calcium phosphate ions to enter the deeper regions, resulting in suboptimal blocking effect [103]. PAMAM-induced biomineralization showed excellent stable occlusion effect even after acid challenge, highlighting great potential for use in the treatment of dentin hypersensitivity reactions.
Dentin remineralization studies have shown that both Mesoporous Bioactive Glass Nanoparticles (MBN) and PAMAM are effective in the treatment of DH and have their characteristics. BAG induces the remineralization of the dentin outer disc by releasing ions to form a hydroxyl carbonate layer, to realize the mechanical sealing of dentin tubules [104,105]. However, the time dependence of mineralization makes it difficult to deal with initial DH quickly. To overcome this limitation, Bad et al coated the surface of MBN with PAMAM to form PAMAM-MBN, which has a sealing effect and fills the gaps between the MBN pores in the PAMAM@MBN for better occlusion of dentin tubules as compared to MBN alone. Importantly, the PAMAM coating does not interfere with calcium ion release from MBN. The combined application of PAMAM-MBN has higher PAMAM reactivity and ion release effect of MBN, effectively sealing dentinal tubules at an early stage and thus offering potential for improving initial symptoms associated with dentin hypersensitivity [106]. Bacterial acid production is one of the most prominent pathological causes of caries, and therefore the treatment of caries requires not only remineralization therapy but also concomitant antimicrobial therapy. PAMA-NH2 is inherently antimicrobial, with a large number of positive charges on its externally protonated amino-terminal group, which confers a strong affinity to negatively charged bacterial surfaces through electrostatic interactions and is capable of attaching to and puncturing the bacterium, leading to cytoplasmic component leakage and bacterial death [61,107,108]. The growth of PAMAM-NH2 firmly bound to the dentin surface showed good inhibition of Streptococcus mutans, Actinomyces naeslundii, and Enterococcus faecalis, and competitive chelation of calcium and zinc particles to inhibit the activity of matrix metalloproteinase, which is essential for maintaining the durability of resin dentin bonding [105]. Ge et al combined PAMAM and Dimethylamino Dodecyl Methacrylate (DMADDM) to prepare a new type of anticaries adhesive, which played the dual role of natural bio natural mineralization and antimicrobial effect. The incorporation of 1% PAMAM and 5% DMADDM can inhibit Streptococcus mutans, Streptococcus sanguinis, and Streptococcus gordonii, showing superior antibacterial and remineralization properties without compromising the bond strength of the adhesives, which has a promising potential for clinical applications in caries prevention [109]. Zhou et al reported that triclosan-loaded PAMAM-COOH could induce in situ remineralization on of dentin and at the same time release triclosan for a long period for local antimicrobial treatment [110]. Zhu et al. prepared PAMAM-COOH carry with Apigenin (API), and dendrimers rich in API were tightly bound to dentin and induced dentin tubule occlusion. Additionally, the controlled release of apigenin prevents further bacterial erosion of dentin [111]. Dendrimers show great potential as dental caries restoration materials, not only as carriers for capsule release of antimicrobial drugs for local treatment but also as templates for inducing in-situ remineralization. Xiao et al. developed a bioactive multifunctional composite material called BMC, which contains NAPC, Dimethylamino Hexadecyl Methacrylate (DMADHM), 2-Methacryloyloxyethyl Phosphorylcholine (MPC) and silver Nanoparticles (NAg). The additions of DMADHM, MPC, and NAg significantly enhanced the protein-repellent and antibacterial properties of the material, and combined with G3-PAMAM-NH2 joint application achieved maximum mineralization of human root dentin in the artificial salivary lactic acid cycle. The hardness of root dentin reached 0.55±0.04 GPa after 21 days, which close to the hardness of sound root dentin, suggesting that PAMAM+MPC can be used in restorative treatments for the treatment of caries class V cavities on the root surface in order to mineralize and protect the tooth structure [112]. It is worth noting that rapid deposition of minerals on the surface of demineralized teeth may lead to hyper mineralization, which may prevent mineral deposition into the deeper layers of dentin.
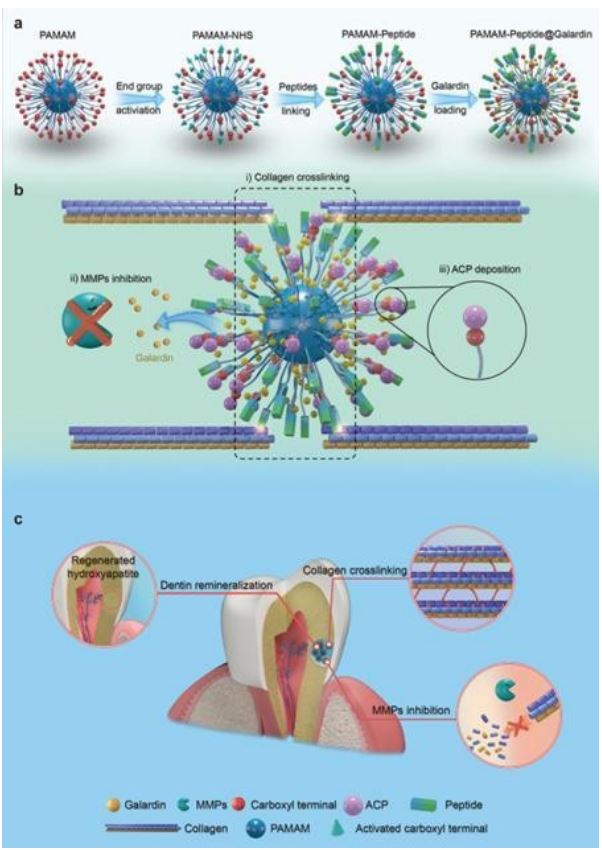
The structural integrity and stability of the collagen matrix are a prerequisite for the effective induction of mimetic remineralization. Under pathological conditions such as dental caries and caries, repeated low pH stimulation activate caries matrix Metalloproteinase (MMPs), leading to the breakdown of collagen protofibrils. However, collagen stability is a major obstacle affecting the clinical application of remineralization templates. PAMAM-OH inhibits exogenous and endogenous MMP activity and prevents the degradation of collagen fibers in the mixed layer, which lays the foundation for the internal mineralization of collagen fibers in the mixed layer induced by PAMAM-OH and realized the persistence of dentin adhesion of the resins [113]. On the other hand, PAMAM-COOH combined with dentin collagen through electrostatic interaction without changing the structure and mechanical properties of dentin collagen, competitively chelating zinc and calcium ions, effectively inactivates endogenous MMPs, and delaying the degradation of collagen by MMPs enzyme. Importantly, PA-MAM-COOH has no adverse effect on the embedding of resin dentin but enhances the adhesion durability of resin dentin. This study provides a theoretical basis for the further clinical application of PAMAM-COOH in etch and rinse adhesive system [114]. Another study stated that PAMAM-COOH had an excitatory effect on free MMP-2 activity, while it did not have any inhibitory effect on dentin-bound MMPs activity. This phenomenon can be attributed to two reasons: (1) the negatively charged PAMAM-COOH is unable to bind to the catalytic site of MMP-2 containing the negatively charged glutamic acid residue, resulting in reduced MMP-2 activity. (2) PAMAM-COOH might play a catalytic role in enhancing the interaction between MMP-2 and FRET peptide [115]. Zhang et al. constructed NPCs-derived collagen cross-linking material, PAMAM-peptide@galardin (PNG), inspired by natural collagen cross-linking agents, by dual modification of PAMAM using an external functional group and an internal drug. PNG was firstly enriched around collagen by peptides, which acted as “bridges” connecting multiple collagen molecules and promoted collagen cross-linking. On this basis, galardin was released continuously to continuously inhibit the activity of MMPs. Moreover, the retained PAMAM carboxyl group attracts ACP deposition on the collagen scaffold, enabling biomimetic remineralization of dentin collagen. These modifications confer PAMAM with the ability to cross-link collagen and inhibit MMPs, so as to realize effective biomimetic remineralization of dentin in an MMPscontaining environment. Animal experiments have also demonstrated that PNG is effective in counteracting rat dentin caries (Figure 6) [116].
Xiang et al synthesized a composite material PAMAM-COOH and MMPs inhibitor Chlorhexidine Gluconate (CG), and the resin and demineralized dentin surface were separated by a 50 μm sheet, with both ends fixed using light curing with flowing resin to simulate a microenvironment with a 50 μm gap for microleakage. Under the gap of 50 μm, the microenvironment may cause localion concentration to be higher than that formed on the external or free surface, and exceed the dissolution product constants of many mineral forms, such as calcium phosphate and calcium fluoride. After soaking in artificial saliva for 14 days, corn-like crystal structures gathered around dentinal tubules, and all exhibiting strong characteristic peaks associated with phosphate compounds.
PAMAM-CG induced dentin remineralization [117]. Cai et al. proposed a novel method to improve bond durability using CHX-loaded PAMAM dendrimer as a pretreatment agent and ACP nanoparticles as a bond filler. PAMAM loaded with CHX can rely on the size repulsion effect to remain in collagen fibers for greater inhibition of MMP activity, and CAP nanoparticle fillers can achieve dentin biomimetic mineralization in the absence of calcium phosphorus particles. PAMAM acts as both a carrier for CHX and reduces the cytotoxicity of CHX, as well as a template for bionic mineralization that induces calcification of CAP-containing adhesives, an approach that effectively improves bond durability [118].
Conclusion
PAMAM plays a crucial role in the process of inducing biomimetic mineralization of tooth tissue: (1) Stabilize calcium and phosphorus ions in the solution and inhibit early spontaneous precipitation; (2) Stabilize ACP and deliver it to the crystal surface of demineralized dental tissue; (3) Provide nucleation sites and mineralization templates for crystals growth, and control the formation of the crystals in an orderly manner. PAMAM has a remarkable ability to induce the mineralization of dental tissues, and the structure, composition, morphology, and arrangement of the formed mineral crystals are close to that of natural dental tissues, which largely simulates the mineralization process of natural dental tissues and restores the structure and properties of the natural dental tissues to a certain extent. However, at present, most of the research has been carried out in a liquid environment in vitro, fewer studies have been conducted in the oral cavity, and there is insufficient evidence to show that good results can be achieved in the oral cavity. Moreover, achieving precise crystal alignment between largesized crystal blocks is currently challenging. The mechanisms underlying crystal orientation and the role and evolution of the organic matrix during mineralization remain incompletely understood. Although PAMAM-induced dental tissues are similar to the normal structure, the study of the physical and chemical properties (microhardness, abrasion resistance, mechanical properties, acid resistance, etc) of induced dental tissues is still in the primary stage. The biomimetic mineralization of tooth tissues needs to be combined with in vitro and in vitro research to 646 further shorten the mineralization time in order to improve the mineralization degree and biological activity. In addition, the biosafety of PAMAM is of concern, and further in vivo and long-term clinical long-technical studies are required to demonstrate the efficacy of long-term use of PAMAM and the adverse effects of long-term use of PAMAM. The solution to these problems still needs a lot of research support, but there is no denying that PAMAM has significant potential in the biomimetic mineralization of tooth hard tissues.
Declarations
Conflicts of interest: The authors declare no conflict of interest.
Author contributions: Conceptualization, S.L., L.Q. and Y.F.; investigation, W.L. and Z.L.; writing original draft preparation, S.L., writing review and editing, Z.L., J.R. and H.Z.; visualization, H.Z., J.R. and LQ, supervision, Y.F., C.K. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding: This study was funded by Science and Technology Department of Jilin Province (Grant No.20230204076YY, 20220401102YY, 20210204128YY). This study was funded by National Nature Science Foundation of China (Grant No. 82370934). This study was funded by Changchun Science and Technology Bureau (Grant No. 18YJ010). This study was funded by Project of Rural and Social Development Department of Changchun Science and Technology Bureau (Grant No. 21ZGY01).
This study was funded by Major special project of Changchun Science and Technology Bureau (Grant No. 18YJ010). This study was funded by Industrial Technology Research and Development Project of Jilin Provincial Development and Reform Commission (Grant No. 2021C043-2).
Acknowledgments: We would like to express our appreciation to everyone who was involved in the drafting and preparation of the manuscript.
Data availability statement: The datasets [GENERATED/ANALYZED] for this study can be found in the [NAME OFREPOSITORY] [LINK]. Please see the “Availability of data” section of Materials and data policies in the Author guidelines for more details.
References
- Williams DM. The research agenda on oral health inequalities: The IADR-GOHIRA initiative.Medical principles and practice: International journal of the Kuwait University, Health Science Centre. 2014; 23(Suppl 1): 52-9.
- Goettsche ZS, Ettinger RL, Wefel JS, Hogan MM, Harless JD, Qian F. In vitro assessment of 3 dentifrices containing fluoride in preventing demineralization of overdenture abutments and root surfaces. The Journal of prosthetic dentistry. 2014; 112(5): 1257-64.
- Besinis A, De Peralta T, Tredwin CJ, Handy RD. Review of nanomaterials in dentistry: Interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS nano. 2015; 9(3): 2255-89.
- Iijima M, Moradian-Oldak J. Control of apatite crystal growth in a fluoride containing amelogenin-rich matrix. Biomaterials. 2005; 26(13): 1595-603.
- Moran GP, Zgaga L, Daly B, Harding M, Montgomery T. Does fluoride exposure impact on the human microbiome? Toxicology letters. 2023; 379: 11-9.
- Ren C, Li HH, Zhang CY, Song XC. Effects of chronic fluorosis on the brain. Ecotoxicology 74 and environmental safety. 2022; 244: 114021.
- Kim YK, Yiu CK, Kim JR, Gu L, Kim SK, et al. Failure of a glass ionomer to remineralize apatite-depleted dentin. Journal of dental research. 2010; 89(3): 230-5.
- Liu Y, Kim Y-K, Dai L, Li N, Khan SO, et al. Hierarchical and non-hierarchical mineralisation of collagen. Biomaterials. 2011; 32(5): 1291-300.
- Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008; 29(8): 1127-37.
- Mai S, Kim YK, Kim J, Yiu CK, Ling J, et al. In vitro remineralization of severely compromised bonded dentin. Journal of dental research. 2010; 89(4): 405-10.
- Mai S, Kim YK, Toledano M, Breschi L, Ling JQ, et al. Phosphoric acid esters cannot replace polyvinylphosphonic acid as phosphoprotein analogs in biomimetic remineralization of resin bonded dentin. Dental materials: Official publication of the Academy of Dental Materials. 2009; 25(10): 1230-9.
- Pouget E, Dujardin E, Cavalier A, Moreac A, Valéry C, et al. Hierarchical architectures by synergy between dynamical template self-assembly and bio mineralization. Nature materials. 2007; 6(6): 434-9.
- Prasad M, Butler WT, Qin C. Dentin sialo phosphoprotein in bio mineralization. Connective tissue research. 2010; 51(5): 404-17.
- Svenson S, Tomalia DA. Dendrimers in biomedical applicationsreflections on the field. Advanced drug delivery reviews. 2005; 57(15): 2106-29.
- Kheraldine H, Rachid O, Habib AM, Al Moustafa AE, Benter IF, et al. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Advanced drug delivery reviews. 2021; 178: 113908.
- Tomalia Da, Baker H, Dewald J, Hall M, Kallos G, et al. A New Class of Polymers: Starburst-Dendritic Macromolecules Polymer Journal. 1985; 17(1): 117-32.
- Filipczak N, Yalamarty SSK, Li X, Parveen F, Torchilin V. Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules (Basel, Switzerland). 2021; 26(11).
- Kharwade R, More S, Warokar A, Agrawal P, Mahajan N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arabian Journal of Chemistry. 2020; 13(7): 6009-39.
- Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. Journal of internal medicine. 2014; 276(6): 579-617.
- Araújo RV, Santos SDS, Igne Ferreira E, Giarolla J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules (Basel, Switzerland). 2018; 23(11).
- Wu D, Chen X, Chen T, Ding C, Wu W, et al. Substrate-anchored and degradation-sensitive anti-inflammatory coatings for implant materials. Scientific reports. 2015; 5: 11105.
- Moradian-Oldak J. Amelogenins: Assembly, processing and control of crystal morphology. Matrix biology: journal of the International Society for Matrix Biology. 2001; 20(5-6): 293-305.
- Wang Y, Guo R, Cao X, Shen M, Shi X. Encapsulation of 2-methoxyestradiol within multifunctional poly(amidoamine) dendrimers for targeted cancer therapy. Biomaterials. 2011; 32(12): 3322-9.
- Tarach P, Janaszewska A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. International journal of molecular sciences. 2021; 22(6).
- Luong D, Kesharwani P, Deshmukh R, Mohd Amin MCI, Gupta U, et al. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug an gene delivery. Acta biomaterialia. 2016; 43: 14-29.
- Vu MT, Bach LG, Nguyen DC, Ho MN, Nguyen NH, et al. Modified Carboxyl-Terminated PAMAM Dendrimers as Great Cytocompatible Nano-Based Drug Delivery System. International journal of molecular sciences. 2019; 20(8).
- Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, et al. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. Journal of controlled release: official journal of the Controlled Release Society. 2000; 65(1-2): 133-48.
- Fox LJ, Richardson RM, Briscoe WH. PAMAM dendrimer - cell membrane interactions. Advances in colloid and interface science. 2018; 257: 1-18.
- Naka K, Tanaka Y, Chujo Y. Effect of Anionic Starburst Dendrimers on the Crystallization of CaCO3 in Aqueous Solution: Size Control of Spherical Vaterite Particles. Langmuir: The ACS journal of surfaces and colloids. 2002; 18(9): 3655-8.
- Tanaka Y, Nemoto T, Naka K, Chujo Y. Preparation of CaCO3/polymer composite films via interaction of anionic starburst dendrimer with poly(ethylenimine). Polymer Bulletin. 2000; 45: 447- 802 50.
- Zhang F, Yang SP, Chen HM, Wang ZH, Yu XB. The effect of an anionic starburst dendrimer on the crystallization of BaWO4 under hydrothernial reaction conditions. Journal of Crystal Growth. 2004; 267(3-4): 569-73.
- Yan SJ, Zhou ZH, Zhang F, Yang SP, Yang LZ, et al. Effect of anionic PAMAM with amido groups starburst dendrimers on the crystallization of Ca-10(PO4)(6)(OH)(2)) by hydrothermal method. Materials Chemistry and Physics. 2006; 99(1): 164-9.
- Lungu A, Rusen E, Butac LM, Stancu IC. Epoxy-mediated immobilization of pamam dendrimers on methacrylic hydrogels. Digest Journal of Nanomaterials and Biostructures. 2009; 4(1): 97-107.
- Zhang F, Zhou ZH, Yang SP, Mao LH, Chen HM, et al. Hydrotherynal synthesis of hydroxyapatite nanorods in the presence of anionic starburst dendrimer. Materials Letters. 2005; 59(11): 1422-5.
- Khopade AJ, Khopade S, Jain NK. Development of hemoglobin aquasomes from spherical hydroxyapatite cores precipitated in the presence of half-generation poly (amidoamine) dendrimer. International Journal of Pharmaceutics. 2002; 241(1): 145-54.
- Zhou Z-H, Zhou P-L, Yang S-P, Yu X-B, Yang L-Z. Controllable synthesis of hydroxyapatite nanocrystals via a dendrimer-assisted hydrothermal process. Materials Research Bulletin. 2007; 42(9): 1611-8.
- Kawai T, Ohtsuki C, Kamitakahara M, Miyazaki T, Tanihara M, et al. Coating of an apatite layer on polyamide films containing sulfonic groups by a biomimetic process. Biomaterials. 2004; 25(19): 4529-34.
- Xie L, Wang L, Jia X, Kuang G, Yang S, et al. Effects of glutamic acid shelled PAMAM dendrimers on the crystallization of calcium phosphate in diffusion systems. Polymer Bulletin. 2011; 66(1): 119-32.
- Gil-Bona A, Bidlack FB. Tooth Enamel and its Dynamic Protein Matrix. International journal of molecular sciences. 2020; 21(12).
- Tao J, Fijneman A, Wan J, Prajapati S, Mukherjee K, et al. Control of Calcium Phosphate Nucleation and Transformation through Interactions of Enamelin and Amelogenin Exhibits the Goldilocks Effect. Crystal growth & design. 2018; 18(12): 7391-400.
- Moradian-Oldak J, George A. Bio mineralization of Enamel and Dentin Mediated by Matrix Proteins. Journal of dental research. 2021; 100(10): 1020-9.
- Pandya M, Diekwisch TGH. Enamel biomimetics-fiction or future of dentistry. International journal of oral science. 2019; 11(1): 8.
- Mukherjee K, Ruan Q, Nutt S, Tao J, De Yoreo JJ, et al. PeptideBased Bioinspired Approach to Regrowing Multilayered Aprismatic Enamel. ACS omega. 2018; 3(3): 2546-57.
- Lokappa SB, Chandrababu KB, Moradian-Oldak J. Tooth enamel protein amelogenin binds to ameloblast cell membrane-mimicking vesicles via its N-terminus. Biochemical and biophysical research communications. 2015; 464(3): 956-61.
- Bromley KM, Kiss AS, Lokappa SB, Lakshminarayanan R, Fan D, et al. Dissecting amelogenin protein nanospheres: characterization of metastable oligomers. The Journal of biological chemistry. 2011; 286(40): 34643-53.
- Chen CL, Bromley KM, Moradian-Oldak J, DeYoreo JJ. In situ AFM study of amelogenin assembly and disassembly dynamics on charged surfaces provides insights on matrix protein self-assembly. Journal of the American Chemical Society. 2011; 133(43): 17406-13.
- Li QL, Ning TY, Cao Y, Zhang WB, Mei ML, et al. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC biotechnology. 2014; 14: 32
- Tsiourvas D, Tsetsekou A, Kammenou MI, Boukos N. Biomimetic synthesis of ribbon-like hydroxyapatite employing poly(l-arginine). Materials science & engineering C, Materials for biological applications. 2016; 58: 1225-31.
- Jasmine MJ, Prasad E. Fractal growth of PAMAM dendrimer aggregates and its impact on the intrinsic emission properties. The journal of physical chemistry B. 2010; 114(23): 7735-42.
- Yang J, Cao S, Li J, Xin J, Chen X, et al. Staged self-assembly of PAMAM dendrimers into macroscopic aggregates with a microribbon structure similar to that of amelogenin. Soft Matter. 2013; 9(31): 7553-9.
- Yang S, He H, Wang L, Jia X, Feng H. Oriented crystallization of hydroxyapatite by the biomimetic amelogenin nanospheres from self-assemblies of amphiphilic dendrons. Chemical 860 communications (Cambridge, England). 2011; 47(36): 10100-2.
- Xin J, Chen T, Lin Z, Dong P, Tan H, et al. Phosphorylated dendronized poly(amido amine)s as protein analogues for directing hydroxylapatite biomineralization. Chemical communications (Cambridge, England). 2014; 50(49): 6491-3.
- Chen L, Liang K, Li J, Wu D, Zhou X, et al. Regeneration of biomimetic hydroxyapatite on etched human enamel by anionic PAMAM template in vitro. Archives of oral biology. 2013; 58(8): 975-80.
- Chen L, Yuan H, Tang B, Liang K, Li J. Biomimetic remineralization of human enamel in the presence of polyamidoamine dendrimers in vitro. Caries research. 2015; 49(3): 282-90.
- Tantbirojn D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. Journal of dentistry. 2008; 36(1): 74-9.
- Fan M, Zhang M, Xu HHK, Tao S, Yu Z, et al. Remineralization effectiveness of the PAMAM dendrimer with different terminal groups on artificial initial enamel caries in vitro. Dental materials: Official publication of the Academy of Dental Materials. 2020; 36(2): 210-20.
- Weerkamp AH, Uyen HM, Busscher HJ. Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva-coated human enamel and dentin. Journal of dental research. 1988; 67(12): 1483-7.
- Shi X, Thomas TP, Myc LA, Kotlyar A, Baker JR. Jr. Synthesis, characterization, and intracellular uptake of carboxyl-terminated poly (amidoamine) dendrimer-stabilized iron oxide nanoparticles. Physical chemistry chemical physics: PCCP. 2007; 9(42): 5712-20.
- Jia L, Tao S, Yang J, Liang K, Yu Z, et al. Adhesion of Streptococcus mutans on remineralized enamel surface induced by poly(amido amine) dendrimers. Colloids and surfaces B, Biointerfaces. 2021; 197: 111409.
- Cheng Y, Feng G, Moraru CI. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Frontiers in microbiology. 2019; 10: 191.
- Ciolkowski M, Rozanek M, Bryszewska M, Klajnert B. The influence of PAMAM dendrimers surface groups on their interaction with porcine pepsin. Biochimica et biophysica acta. 2013; 1834(10): 1982-7.
- Naka K. Effect of dendrimers on the crystallization of calcium carbonate in aqueous solution. Topics in current chemistry. 2003; 228: 141-58.
- Chen H, Banaszak Holl M, Orr BG, Majoros I, Clarkson BH. Interaction of dendrimers (artificial proteins) with biological hydroxyapatite crystals. Journal of dental research.2003; 82(6): 443-8.
- Kirkham J, Zhang J, Brookes SJ, Shore RC, Wood SR, et al. Evidence for charge domains on developing enamel crystal surfaces. Journal of dental research. 2000; 79(12): 1943-7.
- Chen H, Chen Y, Orr BG, Holl MM, Majoros I, et al. Nanoscale probing of enamel nanorod surface using polyamidoamine dendrimers. Langmuir: the ACS journal of surfaces and colloids. 2004; 20(10): 4168-71.
- Wu D, Yang J, Li J, Chen L, Tang B, et al. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials. 2013; 34(21): 5036-47.
- Chen M, Yang J, Li J, Liang K, He L, et al. Modulated regeneration of acid-etched human tooth enamel by a functionalized dendrimer that is an analog of amelogenin. Acta biomaterialia. 2014; 10(10): 4437-46.
- Gao Y, Liang K, Weir MD, Gao J, Imazato S, et al. Enamel remineralization via poly(amido amine) and adhesive resin containing calcium phosphate nanoparticles. Journal of dentistry. 2020; 92: 103262.
- Tao S, Yang X, Liao L, Yang J, Liang K, et al. A novel anticaries agent, honokiol-loaded poly (amido amine) dendrimer, for simultaneous long-term antibacterial treatment and remineralization of demineralized enamel. Dental materials: Official publication of the Academy of Dental Materials. 2021; 37(9): 1337-49.
- Du T, Niu X, Li Z, Li P, Feng Q, et al. Crosslinking induces high mineralization of apatite minerals on collagen fibers. International journal of biological macromolecules. 2018; 113: 450-7.
- Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, et al. Biomimetic remineralization of dentin. Dental materials: official publication of the Academy of Dental Materials. 2014; 30(1): 77- 914 96.
- Di Foggia M, Prati C, Gandolfi MG, Taddei P. An in vitro study on dentin demineralization and remineralization: Collagen rearrangements and influence on the enucleated phase. Journal of inorganic biochemistry. 2019; 193: 84-93.
- Bacino M, Girn V, Nurrohman H, Saeki K, Marshall SJ, et al. Integrating the PILP-mineralization process into a restorative dental treatment. Dental materials: Official publication of the Academy of Dental Materials. 2019; 35(1): 53-63.
- Retana-Lobo C, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Mendes de Souza BD, Reyes-Carmona J. Non-Collagenous Dentin Protein Binding Sites Control Mineral Formation during the Biomineralisation Process in Radicular Dentin. Materials (Basel, Switzerland). 2020; 13(5).
- Su QQ, Zhang C, Mai S, Lin HC, Zhi QH. Effect of poly (γ-glutamic acid)/tricalcium phosphate (γ-PGA/TCP) composite for dentin remineralization in vitro. Dental materials journal. 2021; 40(1): 26-34.
- Liang K, Xiao S, Shi W, Li J, Yang X, et al. 8DSS-promoted remineralization of demineralized dentin in vitro. Journal of materials chemistry B. 2015; 3(33): 6763-72.
- El Gezawi M, Wölfle UC, Haridy R, Fliefel R, Kaisarly D. Remineralization, Regeneration, and Repair of Natural Tooth Structure: Influences on the Future of Restorative Dentistry Practice. ACS biomaterials science & engineering. 2019; 5(10): 4899-919.
- Gericke A, Qin C, Sun Y, Redfern R, Redfern D, et al. Different forms of DMP1 play distinct roles in mineralization. Journal of dental research. 2010; 89(4): 355-9.
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. The Journal of biological chemistry. 2007; 282(2): 1193-204.
- Nijhuis AW, Nejadnik MR, Nudelman F, Walboomers XF, Te Riet J, et al. Enzymatic pH control for biomimetic deposition of calcium phosphate coatings. Acta biomaterialia. 2014; 10(2): 931-9.
- He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005; 44(49): 16140-8.
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, et al. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. The Journal of biological chemistry. 2004; 279(18): 18115-20.
- Jäger I, Fratzl P. Mineralized collagen fibrils: A mechanical model with a staggered arrangement of mineral particles. Biophysical journal. 2000; 79(4): 1737-46.
- Li J, Yang J, Li J, Chen L, Liang K, et al. Bioinspired intrafibrillar mineralization of human dentine by PAMAM dendrimer. Biomaterials. 2013; 34(28): 6738-47.
- Xie F, Wei X, Li Q, Zhou T. In vivo analyses of the effects of polyamidoamine dendrimer on dentin biomineralization and dentinal tubules occlusion. Dental materials journal. 2016; 35(1): 104-11.
- Takahashi M, Nakajima M, Tagami J, Scheffel DL, Carvalho RM, et al. The importance of size-exclusion characteristics of type I collagen in bonding to dentin matrices. Acta biomaterialia. 2013; 9(12): 9522-8.
- Zhang H, Yang J, Liang K, Li J, He L, et al. Effective dentin restorative material based on phosphate-terminated dendrimer as artificial protein. Colloids and surfaces B, Biointerfaces. 2015; 128: 304-14.
- Qin H, Long J, Zhou J, Wu L, Xie F. Use of phosphorylated PAMAM and carboxyled PAMAM to induce dentin biomimetic remineralization and dentinal tubule occlusion. Dental materials journal. 2021; 40(3): 800-7.
- Liang KN, Yuan H, Li JS, Yang JJ, Zhou X, et al. Remineralization of Demineralized Dentin Induced by Amine-Terminated PAMAM Dendrimer. Macromolecular Materials and Engineering. 2015; 300(1): 107-17.
- Liang K, Gao Y, Li J, Liao Y, Xiao S, et al. Biomimetic mineralization of collagen fibrils induced by amine-terminated PAMAM dendrimers-PAMAM dendrimers for remineralization. Journal of biomaterials science Polymer edition. 2015; 26(14): 963-74.
- Tao SY, Fan ML, Xu HHK, Li JS, He LB, et al. The remineralization effectiveness of PAMAM dendrimer with different terminal groups on demineralized dentin in vitro. Rsc Advances. 2017; 7(87): 54947-55.
- Yang J, Huang J, Qin H, Long J, Lin X, et al. Remineralization of human dentin type I collagen fibrils induced by carboxylated polyamidoamine dendrimer/amorphous calcium phosphate nanocomposite: an in vitro study. Journal of biomaterials science Polymer edition. 2022; 33(5): 668- 86.
- Liang K, Weir MD, Reynolds MA, Zhou X, Li J, et al. Poly (amido amine) and nano- calcium phosphate bonding agent to remineralize tooth dentin in cyclic artificial saliva/lactic acid. Materials science & engineering C, Materials for biological applications. 2017; 72: 7-17.
- Liang K, Weir MD, Xie X, Wang L, Reynolds MA, et al. Dentin remineralization in acid challenge environment via PAMAM and calcium phosphate composite. Dental materials: official publication of the Academy of Dental Materials. 2016; 32(11): 1429-40.
- Liang K, Xiao S, Weir MD, Bao C, Liu H, et al. Poly (amido amine) dendrimer and dental adhesive with calcium phosphate nanoparticles remineralized dentin in lactic acid. Journal of biomedical materials research Part B, Applied biomaterials. 2018; 106(6): 2414-24.
- Liang K, Zhou H, Weir MD, Bao C, Reynolds MA, et al. Poly(amido amine) and calcium phosphate nanocomposite remineralization of dentin in acidic solution without calcium phosphate ions. Dental materials : official publication of the Academy of Dental Materials. 2017; 33(7): 818-29.
- Liang K, Gao Y, Xiao S, Tay FR, Weir MD, et al. Poly(amido amine) and rechargeable adhesive containing calcium phosphate nanoparticles for long-term dentin remineralization. Journal of dentistry. 2019; 85: 47-56.
- Liang K, Xiao S, Wu J, Li J, Weir MD, et al. Long-term dentin remineralization by poly(amido amine) and rechargeable calcium phosphate nanocomposite after fluid challenges. Dental materials: official publication of the Academy of Dental Materials. 2018; 34(4): 607-18.
- Liang K, Gao Y, Tao S, Weir MD, Zhou C, et al. Dentin remineralization in acidic solution without initial calcium phosphate ions via poly(amido amine) and calcium phosphate nanocomposites after fluid challenges. Clinical oral investigations. 2022; 26(2): 1517-30.
- Liang K, Gao Y, Li J, Liao Y, Xiao S, et al. Effective dentinal tubule occlusion induced by polyhydroxy-terminated PAMAM dendrimer in vitro. Rsc Advances. 2014; 4(82): 43496-503.
- Wen Y, Wang J, Luo J, Yang J. Remineralization of dentine tubules induced by phosphate- terminated PAMAM dendrimers. Heliyon. 2020; 6(12): e05886.
- Liu Y, Li N, Qi YP, Dai L, Bryan TE, et al. Intrafibrillar collagen mineralization produced by biomimetic hierarchical nanoapatite assembly. Advanced materials (Deerfield Beach, Fla). 2011; 23(8): 975-80.
- Gao Y, Liang K, Li J, Yuan H, Liu H, et al. Effect and Stability of Poly(Amido Amine)-Induced Biomineralization on Dentinal Tubule Occlusion. Materials (Basel, Switzerland). 2017; 10(4).
- Sukumaran SK, Vadakkekuttical RJ, Kanakath H. Comparative evaluation of the effect of curcumin and chlorhexidine on human fibroblast viability and migration: An in vitro study. Journal of Indian Society of Periodontology. 2020; 24(2): 109-16.
- Gou Y, Jin W, He Y, Luo Y, Si R, et al. Effect of Cavity Cleanser With Long-Term Antibacterial and Anti-Proteolytic Activities on Resin-Dentin Bond Stability. Frontiers in cellular and infection microbiology. 2021; 11: 784153.
- Bae J, Son WS, Yoo KH, Yoon SY, Bae MK, et al. Effects of Poly (Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization. Nanomaterials (Basel, Switzerland). 2019; 9(4).
- Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Molecular pharmaceutics. 2012; 9(3): 342-54.
- Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002; 23(16): 3359-68.
- Ge Y, Ren B, Zhou X, Xu HHK, Wang S, et al. Novel Dental Adhesive with Biofilm-Regulating and Remineralization Capabilities. Materials (Basel, Switzerland). 2017; 10(1).
- Zhou Y, Yang J, Lin Z, Li J, Liang K, et al. Triclosan-loaded poly(amido amine) dendrimer for simultaneous treatment and remineralization of human dentine. Colloids and surfaces B, Biointerfaces. 2014; 115: 237-43.
- Zhu B, Li X, Xu X, Li J, Ding C, et al. One-step phosphorylated poly(amide-amine) dendrimer loaded with apigenin for simultaneous remineralization and antibacterial of dentine. Colloids and surfaces B, Biointerfaces. 2018; 172: 760-8.
- Xiao S, Liang K, Weir MD, Cheng L, Liu H, et al. Combining Bioactive Multifunctional Dental Composite with PAMAM for Root Dentin Remineralization. Materials (Basel,Switzerland). 2017; 10(1).
- Luo Y, Si R, He Y, Wang M, Yu Y, et al. Effect of polyhydroxy-terminated PAMAM dendrimer on dentin matrix metalloproteinases within the hybrid layers. BMC oral health. 2023; 23(1): 141.
- Wu Q, Shan T, Zhao M, Mai S, Gu L. The inhibitory effect of carboxyl-terminated polyamidoamine dendrimers on dentine host-derived matrix metalloproteinases in vitro in an etch-andrinse adhesive system. Royal Society open science. 2019; 6(10): 182104.
- Chen L, Chen W, Yu Y, Yang J, Jiang Q, et al. Effect of chlorhexidine-loaded poly (amido amine) dendrimer on matrix metalloproteinase activities and remineralization in etched human dentin in vitro. Journal of the mechanical behavior of biomedical materials. 2021; 121: 104625.
- Tao S, Yang J, Su Z, Zhou F, Wang Z, et al. A Dentin Biomimetic Remineralization Material with an Ability to Stabilize Collagen. Small (Weinheim an der Bergstrasse, Germany). 2022; 18(38): e2203644.
- Xiang K, Chen L, Chen W, Yang D. Remineralization of dentin induced by a compound of polyamide-amine and chlorhexidine in a resin dentin bonding microenvironment. Annals of translational medicine. 2021; 9(6): 472.
- Cai X, Wang X. Chlorhexidine-loaded poly (amido amine) dendrimer and a dental adhesive containing amorphous calcium phosphate nanofillers for enhancing bonding durability. Dental materials: Official publication of the Academy of Dental Materials. 2022; 38(5): 824-34.