SciBase Journals
SciBase Epidemiology and Public Health
ISSN 2691-7785
- Article Type: Research Article
- Volume 1, Issue 1
- Received: Jul 05, 2023
- Accepted: Jul 24, 2023
- Published Online: Jul 25, 2023
Impact of In-Hospital Hyperglycemia on the Outcome of Patients with COVID-19
Julianna do Amaral Ritter1*; Lucas Pitrez Mocellin2; Ana Letícia Vargas Barcelos3; Josefine Busanello4
1Resident in the Multiprofessional Integrated Residency Program in Urgency and Emergency, Federal University of Pampa, Uruguaiana, Brazil.
2Adjunct Professor of the Medicine Course, Federal University of Pampa, Uruguaiana, Brazil.
3Adjunct Professor of the Nutrition and Food Science and Technology Courses, Federal University of Pampa, Itaqui, Brazil.
4Associate Professor of the Nursing Course, Federal University of Pampa, Uruguaiana, Brazil.
*Corresponding Author: Julianna do Amaral Ritter
Resident in the Multiprofessional Integrated Residency Program in Urgency and Emergency, Federal University of Pampa, Uruguaiana, Brazil.
Email: juliannarit@gmail.com
Abstract
Purpose: To investigate the relationship between in-hospital hyperglycemia and clinical outcomes in hospitalized patients affected by Coronavirus Disease 2019 (COVID-19).
Methods: Retrospective longitudinal study carried out at the Hospital Santa Casa de Uruguaiana, through document analysis of the medical records of patients hospitalized with COVID-19 in the study setting, between the months of August 2020 and August 2021. For the analyses, the admitted patients were divided into two groups, according to the classification of blood glucose. The effects of hyperglycemia on clinical outcomes were verified using bivariate and multivariate analysis, with Odds Ratio (OR) and 95% confidence intervals (95%CI). Adjusted logistic regression models were built with three input levels of covariates. The main outcomes were the need for ventilatory support, admission to an Intensive Care Unit (ICU) and death.
Results: The study consisted of 54 individuals (57% male), with a mean age of 56.5±2.2 years. Blood glucose values ranged from 74 to 569 mg/dL and there was no record of hypoglycemia. Overall, 42.6% of the sample had blood glucose levels above 140 mg/dL. Higher blood glucose values were associated with a greater need for ventilatory support among individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients with blood glucose above 140 mg/dL were predisposed to the need for oxygen (OR: 10.476, p=0.032), and this risk was 24 times higher after adjusting for possible confounders (OR: 24.337; p=0.014). However, no association was found between hyperglycemia and ICU admission or death, even in adjusted models.
Conclusion: In-hospital hyperglycemia is associated with an increased risk of severe respiratory involvement among adult and elderly patients hospitalized with COVID-19. However, high glycemic values were not predictors of composite outcomes, such as ICU admission and/or death.
Keywords: Hyperglycemia; Severe acute respiratory syndrome; SARS-CoV-2; COVID-19; Hospitalization.
Citation: Amaral Ritter J, Pitrez Mocellin L, Vargas Barcelos AL, Busanello J. Impact of In-Hospital Hyperglycemia on the Outcome of Patients with COVID-19. SciBase Epidemiol Public Health. 2023; 1(1): 1001.
Introduction
Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), began in Wuhan, China [1], and is considered one of the most important epidemics of the century [2]. According to the World Health Organization (WHO), globally, COVID-19 infection has exceeded 689.7 million cases and 6.8 million deaths [3]. In Brazil, the disease affected approximately 37.6 million cases; of these, 700,000 resulted in death [4]; and these estimates only increased.
The specific mechanisms and risk factors underlying a more severe clinical manifestation remain unknown. However, since the onset of the disease, numerous predictors of worsening COVID-19 have been identified in observational studies. In certain population groups, mortality and morbidity rates increase significantly, such as men [5], elderly people [6] or individuals with comorbidities, especially diabetes mellitus [7], systemic arterial hypertension [8] and obesity [9].
The relationship between the diagnosis of diabetes and the favoring of some infections is well documented [10]. Influenza and pneumonia seem to occur with greater severity among the population that has diabetes [11,12] and, in the current context, the results complement these findings. The occurrence of COVID-19 in individuals diagnosed with diabetes seems to reflect a higher rate of morbidity and mortality compared to those who do not have this chronic condition [13]. Furthermore, diabetes and persistent hyperglycemia have been reported as significant predictors of worse outcomes in this setting [14,15].
However, the available evidence is controversial. The literature suggests that the worst outcomes of COVID-19 infection are closely related to the presence of diabetes [16,17]; however, new studies argue that persistent hyperglycemia is a predictive factor for disease worsening, regardless of the diagnosis of diabetes [18-20]. Thus, the association between the glycemic profile during hospitalization and the characteristics of the SARS-CoV-2 infection remains uncertain. Therefore, the aim of this study was to investigate the relationship between in-hospital hyperglycemia and clinical outcomes in hospitalized patients affected by COVID-19.
Materials and methods
Study design and sample
This is a retrospective longitudinal study, carried out at Hospital Santa Casa de Uruguaiana, in the state of Rio Grande do Sul, Brazil. This study is linked to the larger project entitled “Clinical and epidemiological profile of patients with COVID-19 and factors related to death and hospital care”
Data collection was carried out through document analysis of the medical records of patients hospitalized in the study setting, with COVID-19 diagnoses, between August 2020 and August 2021. Clinical data available in the system were obtained, including demographic information (gender and age), history of previous disease (diabetes and systemic arterial hypertension), in-hospital blood glucose, need for ventilatory support, transfer to another institution, hospital discharge, death and length of stay (in clinical inpatient unit, intensive care unit and total time of hospitalization).
The sample attend to the inclusion criteria: hospital care for a confirmed case of COVID-19, established by a rapid test and, subsequently, by the detection of viral RNA of SARS-CoV-2 in nasopharyngeal swab samples (RT-PCR) (ICD- 10 U07.1 or ICD10 U07.2); age equal to or greater than 18 years. Those who remained under observation for less than 24 hours and were released for treatment or home isolation, transferred to another hospital or who died were excluded from the study.
For this study, data from critical and non-critical patients, over 18 years old, affected by COVID-19, treated at the emergency room of this institution and who had blood glucose data available, were used.
Disease severity
Disease severity (critical or non-critical) was assessed from admission to hospital discharge or death. Individuals who progressed to any of the outcomes during hospitalization were classified as critically ill: (1) respiratory failure requiring ventilatory support; (2) organic failures requiring monitoring and treatment in an intensive care unit (ICU); and (3) death [21].
Glycemia
The blood glucose values verified during hospital care, through the capillary blood glucose test or by blood collection and laboratory analysis, were considered. In-hospital blood glucose was classified considering the guidelines of the American Diabetes Association (ADA) [22] and the Brazilian Society of Diabetes (SBD) [23], in accordance with the proposal by the American Association of Clinical Endocrinologists (AACE) [24], being defined as: hypoglycemia, when <70 mg/dL; normoglycemia, when >70 mg/dL and <140 mg/dL; and hyperglycemia, when >140 mg/dL. For the analyses, the patients were divided into two groups: Group 1: individuals with blood glucose levels below 140 mg/dL; group 2: Individuals with blood glucose levels equal to or greater than 140 mg/dL [23,25].
Clinical outcomes
Composite outcome was used to verify the association between blood glucose and the risk of progression to critical cases or death. The composite endpoint consists of progression to any of the following outcomes: hospital discharge, need for ventilatory support, transfer to the ICU, and death.
Statistical analysis
All variables were tested for normality using the Kolmogorov-Smirnov test. Descriptive statistics were used to characterize the study sample. Quantitative variables were described as mean and Standard Deviation (SD) and categorical variables were described as frequencies and percentages. Differences between groups were verified using Student’s t test, when quantitative variables, and Pearson’s chi-square test, when categorical variables. All estimates were stratified according to blood glucose classification.
Associations between exposure variables and clinical outcomes were verified using bivariate analysis with Odds Ratio (OR) and 95% confidence intervals (95%CI). To assess the effects of hyperglycemia on clinical outcomes, multivariate logistic regression was performed, presented by Odds Ratio (OR) and 95%CI. Fitted models were constructed with three input levels of covariates. The first model was adjusted for gender and age; in the second model, the previous variables were maintained in the model and diabetes mellitus and arterial hypertension were included; and the third model included the previous variables plus the length of stay in the Clinical Inpatient Unit (CIU) and in the intensive care unit. All adjustment variables were chosen according to the literature [26] and variables that obtained p<0.1 in the crude analysis were included.
Results
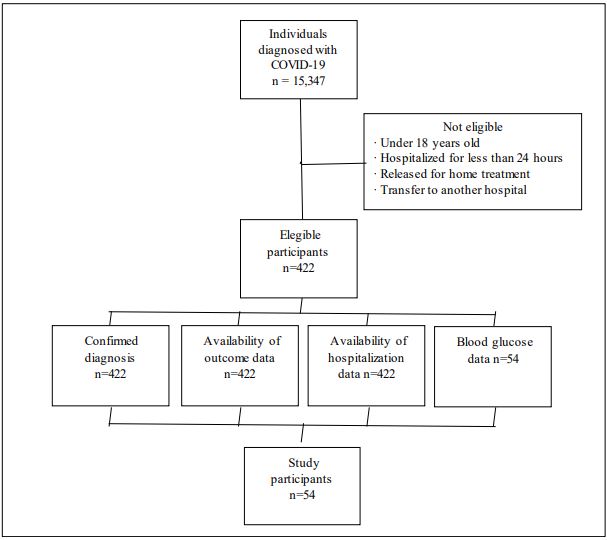
During the study period, 422 subjects met the eligibility criteria. However, only 12.8% (n=54) of this population had blood glucose data recorded, as shown in Figure 1.
The study included 54 individuals (57% male), with a mean age of 56.5±2.2 years. Blood glucose values ranged from 74 to 569 mg/dL and there was no record of hypoglycemia. Arterial hypertension was the most frequent comorbidity (46.3%, n=25), followed by diabetes (29.6%, n=16). The mean length of stay was 5.9±0.6 days. During hospitalization, most of the sample required ventilatory support (79.6%, n=43), with predominance among individuals with glycemic values equal to or greater than 140 mg/dL (p=0.012). Overall, 18 (33.3%) individuals were transferred to the ICU and 12 (22.2%) resulted in death. The characteristics of the sample, according to the classification of in-hospital blood glucose values, are shown in Table 1.
Table 1: Characteristics of study participants according to in-hospital blood glucose classification. Uruguaiana, Rio Grande do Sul, Brazil, 2022. (n=54).
| Variables | All (n=54) | Group 1 (n=31) | Group 2 (n=23) | p |
|---|---|---|---|---|
| Sex, n (%) | 0.075 | |||
| Male | 31 (57.4) | 21 (67.7) | 10 (48.5) | |
| Female | 23 (42.6) | 10 (32.3) | 13 (56.5) | |
| Age, years, mean (±SD) | 56.6 (±2.2) | 54.8 (±3.3) | 59.0 (±2.9) | 0.354 |
| Glucose, mg/dL, mean (±SD) | 174.1 (±14.1) | 111.2 (±3.9) | 259.0 (±22.8) | <0.001 |
| Comorbidities, n (%) | ||||
| Systemic arterial hypertension | 25 (46.3) | 11 (35.5) | 14 (60.9) | 0.064 |
| Diabetes mellitus | 16 (29.6) | 5 (16.1) | 11 (47.8) | 0.012 |
| Disease severity, n (%) | 0.503 | |||
| Critical | 23 (42.6) | 12 (38.7) | 11 (47.8) | |
| Non-critical | 31 (57.4) | 19 (61.3) | 12 (52.2) | |
| Period of hospitalization | ||||
| Total length of stay, days, mean (±SD) | 5.9 (±0.6) | 6.1 (±0.8) | 5.7 (±0.9) | 0.739 |
| Length of stay in CIU >7 days, n (%) | 13 (24.1) | 8 (25.8) | 5 (21.7) | 0.730 |
| Length of stay in the ICU >5 days, n (%) | 11 (20.4) | 7 (22.6) | 4 (17.4) | 0.640 |
| Clinical outcomes | ||||
| Need for ventilatory support, n (%) | 43 (79.6) | 21 (67.7) | 22 (95.7) | 0.012 |
| ICU admission, n (%) | 18 (33.3) | 10 (32.3) | 8 (34.8) | 0.846 |
| Death, n (%) | 12 (22.2) | 8 (25.8) | 4 (17.4) | 0.462 |
Group 1: Participants with glucose levels below 140 mg/dL; Group 2: Participants with glucose levels equal to or greater than 140 mg/dL; SD: Standard Deviation; BMI: Body Mass Index; CIU: Clinical Inpatient Unit; ICU: Intensive Care Unit.
Mean glucose was log-transformed and presented as geometric mean and standard deviation.
Table 2 presents the bivariate analysis performed to verify associations between exposure variables and clinical outcomes. It was observed that blood glucose values above 140 mg/dL represent a greater chance of needing ventilatory support during hospitalization (OR: 10.476, p=0.032). Hospitalization longer than 7 days in a clinical inpatient unit seems to reflect a greater risk of transfer to the intensive care unit (OR: 8.000, p=0.003) and death (OR: 8.400, p=0.004); while hospitalization for more than 5 days in the ICU seems to increase the chances of death among this population (OR: 7.400; p=0.008).
Table 2: Bivariate analysis with odds ratio (OR) and 95% confidence interval (95%CI) of exposure variables, including different in-hospital blood glucose levels, for clinical outcomes. Uruguaiana, Rio Grande do Sul, Brazil, 2022 (n=54).
| Variables | Need for ventilatory support (n=43) | ICU admission (n=18) | Death (n=12) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Glucose <140 mg/dL | 1.0 | 1.0 | 1.0 | ||||||
| Glucose >140 mg/dL | 10.476 | 1.232; 89.116 | 0.032 | 1.120 | 0.358; 3.508 | 0.846 | 0.605 | 0.158; 2.324 | 0.464 |
| Male | 1.385 | 0.353; 5.441 | 0.640 | 0.795 | 0.251; 2.551 | 0.697 | 0.952 | 0.259; 3.495 | 0.941 |
| Age >60 years | 0.864 | 0.228; 3.275 | 0.830 | 0.795 | 0.251; 2.521 | 0.697 | 3.600 | 0.927; 13.971 | 0.064 |
| Systemic arterial hypertension | 1.670 | 0.426; 6.549 | 0.462 | 0.636 | 0.201; 2.012 | 0.442 | 1.867 | 0.509; 6.851 | 0.347 |
| Diabetes mellitus | 0.677 | 0.167; 2.740 | 0.585 | 0.354 | 0.086; 1.455 | 0.150 | 1.250 | 0.316; 4.940 | 0.750 |
| Length of stay in CIU >7 days | 1.547 | 0.289; 8.286 | 0.610 | 8.000 | 1.991; 32.142 | 0.003 | 8.400 | 1.997; 35.336 | 0.004 |
| Length of stay in the ICU >5 days | 7.400 | 1.706; 32.094 | 0.008 | ||||||
OR: Odds Ratio; 95%CI: 95% Confidence Interval; BMI: Body Mass Index; CIU: Clinical Inpatient Unit; ICU: Intensive Care Unit.
Table 3: Multivariate logistic regression with odds ratio (OR) and 95% confidence interval (95%CI) to verify the relationship between in- hospital blood glucose and clinical outcomes. Uruguaiana, Rio Grande do Sul, Brazil, 2022 (n=54).
| Variables | Need for ventilatory support (n=43) | ICU admission (n=18) | Death (n=12) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Glucose <140 mg/dL | 1.0 | 1.0 | 1.0 | ||||||
| Glucose >140 mg/dL | 10.476 | 1.232; 89.116 | 0.032 | 1.120 | 0.358; 3.508 | 0.846 | 0.605 | 0.158; 2.324 | 0.464 |
| Model 1: adjusted for sex and age | |||||||||
| Glucose <140 mg/dL | 1.0 | 1.0 | 1.0 | ||||||
| Glucose >140 mg/dL | 11.642 | 1.283; 105.606 | 0.029 | 1.239 | 0.379; 4.056 | 0.723 | 0.498 | 0.119; 2.082 | 0.339 |
| Model 2: adjusted for variables in model 1 plus systemic arterial hypertension and diabetes mellitus | |||||||||
| Glucose <140 mg/dL | 1.0 | 1.0 | 1.0 | ||||||
| Glucose >140 mg/dL | 19.974 | 1.664; 239.727 | 0.018 | 1.788 | 0.487; 5.570 | 0.381 | 0.519 | 0.113; 2.382 | 0.399 |
| Model 3: adjusted for variables in model 2 plus length of stay in CIU and ICU | |||||||||
| Glucose <140 mg/dL | 1.0 | 1.0 | 1.0 | ||||||
| Glucose >140 mg/dL | 24.337 | 1.904; 311.010 | 0.014 | 3.235 | 0.522; 20.058 | 0.207 | 0.537 | 0.092; 3.126 | 0.489 |
OR: Odds Ratio; 95%CI: 95% Confidence Interval; BMI: Body Mass Index; CIU: Clinical Inpatient Unit; ICU: Intensive Care Unit.
The results of the multivariate logistic regression to verify the relationship between in-hospital blood glucose and clinical outcomes are shown in Table 3. In the first model, adjustments were made for gender and age, and patients with blood glucose above 140 mg/dL showed they were more likely to use ventilatory support during hospitalization (OR: 11.642, p=0.029). In the second model, the previous variables and chronic health conditions were added: diabetes and high blood pressure. In this model, the presence of hyperglycemia also represented a higher risk of respiratory failure (OR: 19.974, p=0.018). In the third model, all the above variables plus hospitalization time greater than 7 days in the ICU and 5 days in the ICU were considered. When compared to normoglycemic individuals, patients with blood glucose above 140 mg/dL had a 24 times greater risk of needing ventilatory support during hospitalization (OR: 24.337, p=0.014). However, no associations were found between hyperglycemia and the need for ICU admission or death, even after adjustments for possible confounders.
Discussion
The present study investigated the relationship between inhospital hyperglycemia and clinical outcomes in hospitalized patients affected by COVID-19 admitted to Hospital Santa Casa de Uruguaiana. The results showed that blood glucose levels greater than 140 mg/dL were independently associated with an increased risk of needing ventilatory support among patients admitted due to SARS-CoV-2 infection at this institution.
It is not surprising that hyperglycemia has been associated with worse outcomes in patients with COVID-19 and without a diagnosis of diabetes. The association of in-hospital hyperglycemia with length of stay, morbidity and mortality, especially in critical illnesses, is widely reported [27,28]. Previous studies have already shown the association between high glycemic values and higher mortality rates in Severe Acute Respiratory Syndrome (SARS-CoV-1) and Middle East Respiratory Syndrome (MERS) [29,30].
Our findings are relevant and are in line with evidence available in the literature. The relationship between in-hospital hyperglycemia, independent of diabetes, and progression to severe COVID-19 has been widely documented [26,31,32]. Recent studies demonstrate that, in patients without diabetes, hyperglycemia at the time of diagnosis of COVID-19 is an independent predictor of mortality and favors the occurrence of unfavorable outcomes, such as ICU admission, use of mechanical ventilation and death [20,33].
A nationally representative retrospective cohort conducted in Turkey included 12,000 patients with confirmed SARS-CoV-2 infection but no previous diagnosis of diabetes, and observed that glucose levels greater than 140 mg/dL increased mortality by 2 .7 times and the rate of ICU admission and/or mechanical ventilation by 2.3 times compared to normoglycemia [33]. The same has been verified in observational studies conducted in European countries, such as Italy and France [19,34].
Several studies have been carried out in order to explain the interaction between blood glucose and the outcomes of SARSCoV-2 infection, especially among the Chinese population. In a study carried out by Zhang et al. [35], 461 adult patients with COVID-19 were included, and the results indicated that hyperglycemia (any glucose level greater than 140 mg/dL during hospitalization) was positively associated with higher inflammatory levels and progression to COVID-19 serious. Additionally, Wang et al. [15], when evaluating 605 adult individuals, found that glycemic values above 126 mg/dL were predictors of mortality among this population.
Another large retrospective longitudinal study was carried out by Bode et al. [18]. The authors analyzed approximately 1122 adult individuals affected by COVID-19, admitted to 88 hospitals in the United States, in order to describe the clinical characteristics of the infection in this population. The main results highlighted persistent hyperglycemia as an independent risk factor for higher rates of hospital complications, such as the need for ventilatory support, mainly the use of mechanical ventilation, ICU admission and death. Additionally, the large study by Carrasco-Sanchez et al. [36] served as a complement to the previously described evidence. In this study, researchers included more than 11,000 Spanish patients and observed that hyperglycemia is related to poor prognosis and mortality in patients infected with SARS-CoV-2, especially with regard to the use of ventilatory support [36] Studies carried out with different populations have found similar results [37-41].
The mechanism of interaction between glucose metabolism and SARS-CoV-2 remains unclear. Recent studies assess the existence of a bidirectional relationship between hyperglycemia and COVID-19. The most common speculations suggest that SARS-CoV-2 uses Angiotensin-Converting Enzyme 2 (ACE2) receptors for entry into cells [42] and, as these receptors are also present in more organs responsible for glucose homeostasis, it is it is possible that the occurrence of COVID-19 causes persistent hyperglycemia when affecting them [43]. In addition, hyperglycemia in the course of the disease, often observed in severe acute conditions, can also be explained by metabolic stress, an important characteristic of infection by this agent [44].
Elevation of plasma glucose damages organs essential for the homeostatic maintenance of the body, including, in addition to cardiovascular, renal and neuronal complications, pulmonary microangiopathy, compromising alveolar gas exchange, already affected by COVID-19 [2]. In addition, hyperglycemia is also associated with a reduction in the metabolic capacity of erythrocytes to capture and diffuse oxygen to tissues, a process secondary to hemoglobin glycation, modifying the tertiary molecular structure of this oxygen-carrying protein [11,13]. In short, impaired gas exchange in response to higher blood glucose levels seems to reflect worse outcomes in SARS-CoV-2 infection, especially in the use of ventilatory support.
In addition to the available evidence on the glycemic impact of SARS-CoV-2 infection, systematic reviews with meta-analyses, including prospective studies on the interrelationship of diabetes and COVID-19, indicate greater morbidity and mortality in individuals who had diabetes and were hyperglycemic [45,46]. Other studies suggest that, although diabetes does not increase the risk of SARS-CoV-2 infection, its presence is closely related to worse outcomes, especially among individuals with poor glycemic control [46,47].
The idea that individuals with chronic health conditions represent an extremely high risk group for adverse outcomes during eventual infections has been widely discussed [48,49]; therefore, its association with worse outcomes in COVID-19 is not surprising. However, it is important to recognize that most of the available data on the role of diabetes in SARS-CoV-2 infection derives from observational studies, making it difficult to properly address pathogenic inferences [50]. In this scenario, the identification and understanding of risk factors for the progression of the disease caused by SARS-CoV-2 are essential, as they provide important evidence for defining conducts during clinical management.
Despite the consistency of our results with the findings of other studies, some methodological issues need to be reconsidered. Much of the research on the subject used different criteria to identify patients without a diagnosis of diabetes and also delimited different cutoff points for the definition of hyperglycemia. Therefore, the definition of the methods used, especially for identifying non-diabetic patients and defining hyperglycemia levels, may differ from the studies available in the literature.
However, part of our findings differ from those reported by other authors. Associations commonly observed in other studies were not significant in our study. Worse health outcomes have been consistently associated with male gender [5], elderly [6] and individuals with chronic health conditions such as diabetes [7], hypertension [8] and obesity [9]. Furthermore, hyperglycemia was not associated with the need for ICU admission or death, diverging from most data available in the literature [25-28]. This inconsistency in the results reinforces the need for further studies on glycemic values and clinical outcomes in SARS-CoV-2 infection in different contexts and populations.
Limitations
This study had limitations that must be addressed. A study performed with a database constructed from pre-existing records is subject to several biases and residual confounding factors. The accuracy of data collection was a potential limiting factor, since it depends on the professional who completes the medical record in the system during the service. The possibility of having patients diagnosed with diabetes, but without the diagnosis referred to at the time of admission, was also not ruled out. The lack of information regarding the use of medication, lifestyle, complementary laboratory tests and anthropometric data favored the fragility of the associations. As a result of the method of data collection, the diagnosis of diabetes may have been missed in a significant proportion of patients. Also, the lack of recording of glycemic values at the time of hospital care limited the sample size. Additionally, the study population was relatively small, with a limited number of results, represented by very wide confidence intervals, which consequently causes a risk of overfitting the regression models. To try to reduce the risk of developing overfitted models, the number of variables in the multivariate analyzes was restricted.
Strengths
Despite all limitations, our study had strengths. A retrospective longitudinal study allows associations to be suggested and expands the possibilities of analyzing the variables. In addition, data from the reality of patients with COVID-19 hospitalized at Hospital Santa Casa de Uruguaiana were reported. In this context, our results are essential, as they allow the verification of the weaknesses presented during the assistance and enable the notification of these aspects, in order to promote the improvement of the service provided to users of the health system.
Conclusion
In conclusion, available evidence suggests that in-hospital hyperglycemia is associated with an increased risk of severe respiratory involvement among adult and elderly patients hospitalized with COVID-19. However, further investigations are needed to explore unexpected associations and elucidate the effects of hyperglycemia on morbidity, mortality, and length of stay among this population, especially in the presence of chronic health conditions.
Declarations
Ethical standards: This study was approved by the Research Ethics Committee of the Federal University of Pampa (Unipampa), with registration number 30837520.2.3004.5324, under opinion number 4.062.712, on May 25th 2020, in accordance with CNS resolution 466/12 , with an informed consent form waived.
Competing interests and funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020.
- Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: Unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020.
- World Health Organization. WHO Coronavirus Disease Dashboard. 2023.
- Ministry of Health. Health Surveillance Secretariat (SVS): COVID-19 Epidemiological Surveillance Guide. 2023.
- Bellou V, Tzoulaki I, Evangelou E, Belbasis L. Risk factors for adverse clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. Eur Respir J. 2022.
- Sinclair AJ, Abdelhafx AH. Age, frailty and diabetes-triple jeopardy for vulnerability to COVID-19 infection. E Clinical Medicine. 2020.
- Lisco G, De Tullio A, Giagulli VA, et al. Hypothesized mechanisms explaining poor prognosis in type 2 diabetes patients with COVID-19: A review. Endocrine. 2020.
- Tadic M, Saeed S, Grassi G, et al. Hypertension and COVID-19: Ongoing Controversies. Front Cardiovasc Med. 2021.
- Soeroto AY, Soetedjo NN, Purwiga A, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: A systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020.
- Alves C, Casqueiro J, Casqueiro J. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012.
- Thomas S, Ouhtit A, Al Khatib HA, et al. Burden and disease pathogenesis of influenza and other respiratory viruses in diabetic patients. J. Infect. Public Health. 2022.
- Allard R, Leclerc P, Tremblay C, et al. Diabetes and the Severity of Pandemic Influenza A (H1N1) Infection. Diabetes Care. 2010.
- Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia-A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020.
- Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020.
- Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: A multicentre retrospective study. Diabetologia. 2020.
- Roncon L, Zuin M, Rigatelli G, et al. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020.
- Fadini GP, Morieri ML, Longato E, et al. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020.
- Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci. Technol. 2020.
- Coppelli A, Giannarelli R, Aragona M, et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: The Pisa COVID-19 study. Diabetes Care. 2020.
- Sardu C, D’Onofrio N, Balestrieri ML, et al. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia. 2020.
- National Health Commission of the People’s Republic of China. The 7th trial version of diagnosis and treatment scheme for pneumonitis caused by COVID-19 infection (in Chinese). 2020.
- American Diabetes Association. Standards of Medical Care in Diabetes-2020. Abridged for Primary Care Providers. Clin Diabetes. 2020.
- Brazilian Society of Diabetes (SBD). Official Position SBD nº 01/2022- Therapeutic Conduct in Type 2 Diabetes: Algorithm SBD 2022. Brasília: Sociedade Brasileira de Diabetes. 2022.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009.
- Yamada T, Shojima N, Noma H, et al. Glycemic control, mortality, and hypoglycemia in critically ill patients: a systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med. 2017.
- Lazarus G, Audrey J, Wangsaputra VK, et al. High admission blood glucose independently predicts poor prognosis in COVID-19 patients: A systematic review and dose-response metaanalysis. Diabetes Res Clin Pract. 2021.
- Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020.
- Barmanray RD, Cheuk N, Fourlanos S, Greenberg PB, Colman PG, et al. In-hospital hyperglycemia but not diabetes mellitus alone is associated with increased in-hospital mortality in Community-Acquired Pneumonia (CAP): A systematic review and metaanalysis of observational studies prior to COVID-19. BMJ Open Diabetes Res Care. 2022.
- Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N. Clinical determinants of the severity of Middle East Respiratory Syndrome (MERS): A systematic review and meta-analysis. BMC Public Health. 2016.
- Halim AA, Alsayed B, Embarak S, Yaseen T, Dabbous S, et al. Clinical characteristics and outcome of ICU admitted MERS corona virus infected patients. Egypt J Chest Dis Tuberc. 2016.
- Wang W, Shen M, Tao Y, Fairley CK, Zhong Q, et al. Elevated glucose level leads to rapid COVID-19 progression and high fatality. BMC Pulm Med. 2021.
- Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID‐19. J Med Virol. 2021.
- Haymana C, Demirci I, Tasci I. Clinical outcomes of non-diabetic COVID-19 patients with different blood glucose levels: A nationwide Turkish study (TurCoGlycemia). Endocrine. 2021.
- Cariou B, Gourdy P, Hadjadj S, Pichelin M, Wargny M. Diabetes and COVID-19: Lessons from the CORONADO study. Med Mal Metab. 2021.
- Zhang W, Li C, Xu Y, He B, Hu M, et al. Hyperglycemia and correlated high levels of inflammation have a positive relationship with the severity of coronavirus Disease 2019. Mediat Inflamm. 2021.
- Carrasco-Sánchez FJ, López-Carmona MD, Martínez-Marcos FJ, Pérez-Belmonte LM, Hidalgo-Jiménez A, et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: Data from the Spanish SEMI-COVID-19 Registry. Ann Med. 2021.
- López-Valdés JI, Ponce-Mendoza RA, Solís-Barraza M, Juan Luis Trevizo-Díaz, Jesús Roberto Nevarez-Campos. Clinical characteristics related to mortality by COVID-19 in intensive care. Rev Med Inst Mex Seguro Soc. 2022; 60: 249-257.
- Zhu L, She Z, Cheng X, Guo J, Zhang BH, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020.
- Sardu C, D’Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, et al. Outcomes in patients with hyperglycemia affected by Covid-19: Can we do more on glycemic control? Diabetes Care. 2020.
- Zhang Y, Li H, Zhang J, Cao Y, Zhao X, et al. The clinical characteristics and outcomes of diabetes mellitus and secondary hyperglycaemia patients with coronavirus disease 2019: A singlecenter, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020.
- Wu J, Huang J, Zhu G, Wang Q, Qingquan LV, et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: A retrospective cohort study. BMJ Open Diabetes Res Care. 2020.
- Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020.
- Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia. 2020.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020.
- Wu Z, Tang Y, Cheng Q. Diabetes increases the mortality of patients with COVID-19: A meta-analysis. Acta Diabetologica. 2020.
- Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020.
- Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, et al. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev Endocr Metab Disord. 2021.
- Lisco G, De Tullio A, Giagulli VA, Guastamacchia E, De Pergola G, et al. Hypothesized mechanisms explaining poor prognosis in type 2 diabetes patients with COVID-19: A review. Endocrine. 2020.
- Tadic M, Saeed S, Grassi G, Taddei S, Mancia G, et al. Hypertension and COVID-19: Ongoing Controversies. Front Cardiovasc Med. 2021.
- Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, et al. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male. 2020.