SciBase Journals
SciBase Epidemiology and Public Health
ISSN 2996-4555
- Article Type: Research Article
- Volume 2, Issue 2
- Received: Feb 28, 2024
- Accepted: Mar 27, 2024
- Published Online: Mar 29, 2024
Influence of Silver Nanoparticles on Germinated and Microwaved Chia Seeds (Salvia Hispanica L)
Dur-e-Shahwar Sattar1*; Sameen Mubarik2; Javerya Sattar3; Saman Mubarik4
1Department of Food Science and Technology, Faculty of Food Science and Nutrition, Bahauddin Zakariya University, 60700, Multan, Punjab, Pakistan.
2Department of Human Nutrition and Dietetics, Faculty of Food Science and Nutrition, Bahauddin Zakariya University, 60700, Multan, Punjab, Pakistan.
3Senior Registrar, Dow University of Health and Sciences, Karachi, Pakistan.
4Institute of Chemical Sciences, Bahauddin Zakariya University, 60700, Multan, Punjab, Pakistan.
*Corresponding Author: Dur-e-shahwar Sattar
Department of Food Science and Technology, Faculty of Food Science and Nutrition, Bahauddin Zakariya University, 60700, Multan, Punjab, Pakistan.
Email: dsattar@bzu.edu.pk
Abstract
This study thoroughly investigated the impact of 60 ppm silver nanoparticles (AgNPs) on the germination process of chia seeds (Salvia Hispanica L.) and an examination of subsequent modifications induced by microwave treatment on AgNP-exposed seeds. The research findings highlighted a significant enhancement in seed germination percentage peaking at 81.67% on the fifth day particularly when exposed to a concentration of 60 ppm AgNPs. Concurrently, radicle length experienced a substantial increase indicative of improved early seedling growth. Morphological study was also studied by scanning electron microscopy. Results indicated that samples treated with microwave showed shrinkage and the samples treated with germination samples showed expansion. Furthermore, investigation of bioactive compounds indicated increased concentrations of total phenolic content and total flavonoids content in samples germinated with AgNPs simultaneously with increased antioxidant activity. In a nutshell, this study provided a comprehensive understanding of the impacts of 60 ppm AgNPs on germination and microwave treatment on chia seeds. The far-reaching implications span across agriculture, food science, and nutritional domains, providing a strong basis for potential future applications and further scientific examination.
Keywords: Chia seeds; Silver nanoparticles; Germination; Microwave Treatment; Bioactive compounds; Morphological study.
Citation: Sattar DS, Mubarik S, Sattar J, Mubarik S. Influence of Silver Nanoparticles on Germinated and Microwaved Chia Seeds (Salvia Hispanica L). SciBase Epidemiol Public Health. 2024; 2(2): 1023.
Introduction
Nanotechnology is an emerging innovative technique for the creation and application of novel Nanomaterials (NMs) industry and many disciplines of study [1]. Precision farming, smart delivery systems, packaging, food safety, and food processing are all applications of nanotechnology [2]. In the agricultural sector, the effects of AgNPs on seed germination, plant growth, and development under various abiotic environments have been determined [3]. AgNPs outperform other Nanoparticles due to their distinct physicochemical features, which impart antibacterial and antioxidant effects [4]. These physiochemical properties, as well as the synthesis and characterization of AgNPs, are influenced by several variables, including pH, temperature, and incubation time, among others [5]. Chia Seed (Salvia Hispanica L.) A biannually cultivated plant is categorized under the mint family (Labiatae), of Spermatophyta, Kingdom of Plantae [6]. It is historically used by the Aztec and Maya people to make traditional medicine, food, and canvases [7]. Chia seeds are also known as “chia,” “chia sage,” and “Spanish sage” and they are consumed in varieties of food that improves its physicochemical and nutritional properties [8]. Chia seeds could be ingested as whole seed, flour, mucilage (gel), and oil, either alone (in natura), in combination with other foods (yogurts, fruits, salads, and soups), or as an ingredient in processed foods (breads, cakes, whole grain bars, and beverages [9]. Germination is a basic procedure in which water is supplied to the seeds until the embryonic axis develops. It is a procedure that does not require soil or controlled light, temperature, and humidity conditions; it is also affordable and may be performed in a household environment [10]. Although simple, germination needs careful monitoring to provide additional insights into the changes that occur during the process, with the goal of developing the best circumstances that result in a safe and high-quality product [11]. The European Parliament defines sprout as “the product obtained from the germination of seeds and their development in water or another medium, harvested before the development of true leaves and which is intended to be eaten whole, including the seed” [12,13]. Recent research has been conducted on germinated chia seeds since their germination appears to be capable of increasing the nutritional and nutraceutical properties of foods [14,15]. Microwave is used to preserve the functional and qualitative features of the seeds [16-18]. Many researchers have employed various roasting processes to enhance the flavor and organoleptic qualities of various seeds such as walnut flour, chia seeds flour, chickpea flour, and barley flour [19,20]. Microwave roasting conditions, which are typically used to prepare plant product like edible seeds and nuts, can significantly alter the physico-chemical properties of seeds and other plant products, including their color, flavor, fatty acid profile, phytochemicals, and bioactive substances [21]. Thus, the aim of the study is to compare and assess the effects of AgNPs (60 ppm) on chia seed germination and microwaved treatment. Selected concentration was taken as it was already investigated that extreme values of silver nanoparticles are harmful for seeds. And 60ppm is considered the safest concentration for all beans and seeds.
Material and methods
Material: Chia seeds were purchased from a local Market, Multan, Pakistan. Chia seeds were then cleaned by removing dust, stones, and residues.
Silver suspensions preparation: The Polyvinyl Pyrrolidone (PVP)-stabilized silver Nanoparticles (powder form, 100 nm) were obtained from Sigma Aldrich Inc. in the USA. To avoid aggregates, silver Nanoparticles were dissolved by sonication in the distilled water. For the current investigation, a variety of approaches were used to characterize silver Nanoparticles. The effect of AgNO3 on seed germination was studied using a 500 mg L-1 [22].
Germination: Hundred grams of chia seeds were mixed with AgNPs solution and kept for 10 mins. Seeds were washed and spread on the tray. Trays (40 cm in diameter) used in the Chia Seed sprouting studies were placed in the trial areas and covered with non-woven fabric. After every three hours, seeds were sprayed to keep them moist. The temperature of the germination environment was calibrated to room temperature (25±2°C). Seeds were germinated till 2-3 cm length on the 4th day. Five days later, the sprouting result was observed. Chia seeds that had sprouted were measured for percentage germination and root lengths. Then seeds were dried for 2 days in the drier at 40OC. These Chia seeds were ground, gathered and put into PE bags for further analysis [22].
Microwave treatment: In a microwave oven, 100 gm of each control and AgNPs (60 ppm) seed sample were individually roasted for 20 minutes at a high temperature of 230°C using 1200 W of power. Chia seeds were put on a wide-capacity oven pan for each treatment. Before further analysis, the seeds were then ground in a laboratory mill, and the resulting powder was sieved through a 60-mesh screen to form a fine powder [23].
Effect of silver nanoparticles on nutritional composition of sprouted and microwaved chia seeds: The working standard protocols described in Official Methods of Analysis in Association of Official Analytical Chemists (AOAC) were used to determine the proximate nutrients, including crude ash (protocol no.923.03), moisture (protocol no.925.10), fat (protocol no.920.85), protein (protocol no.920.87), and fiber (protocol no.32-10), in the raw samples [24].
Effect of silver nanoparticles on color profile of sprouted and microwaved chia seed: A colorimeter (Hunterlab Color Quest colorimeter) was used to estimate the color profile. A* (greenness/redness), L* (darkness/lightness), and b* (blueness/ yellowness) were used to illustrate the values of color [25].
Determination of phenolic, flavonoids components and antioxidant activity of sprouted and microwaved chia seeds
Extract preparation for bioactive compounds: Using an orbital shaker, 10 g of precisely measured Chia Seed powder was added to 100 ml of 100% (v/v) Methanol and homogenized for 8 hours. The mixture was filtered using Whatman No. 1 filter paper to separate the extract. At 40°C and low pressure, a rotary evaporator (Rotary Vacuum Evaporator, EYELA N.N Series) was employed to evaporate the remaining solvent from the Methanolic extract. The resulting extract was then utilized to calculate the Total Phenolic Content (TPC) and DPPH activity [26].
Determination of total phenolic content: 1 ml of a precisely measured sample extract was diluted in 1 ml of sodium carbonate solution (7.5%) and 1 ml of the Folin-Ciocalteu reagent. A UV-VIS spectrophotometer (UV-Vis 3000, ORI, Germany) was used to measure the solution’s absorbance at 765 nm after it had been allowed to stand for 30 minutes. Mg GAE/g was used to represent the overall phenolic content [27].
Free radical scavenging activity by DPPH determination: Precise 2.9 ml of sample extract was dissolved in 0.1 ml of 0.0 mM DPPH and left at room temperature for 30 min at 23°C in the dark. After 30 minutes, the sample was filtered to measure the absorbance at 517 nm with a spectrophotometer (UV-Vis 3000, ORI, Germany). 2.9 ml of DPPH solution and 0.1 ml of methanol were combined to form a control solution [28]. DPPH activity was measured by using the following equation:
DPPH% = Absorbance of control - Absorbance of sample / Absorbance of control * 100
Determination of total flavonoid content: In a test tube, 5.6 ml of distilled water, 0.2 ml of potassium acetate (1 M), 0.2 ml of AlCl3 (10% solution produced in Methanol), and 1 ml of precisely measured sample extract were dissolved. The solution had been kept at room temperature for 30 minutes. Then, using a UV-Vis spectrophotometer (UV-Vis 3000, ORI, Germany), absorbance was determined at 415 nm. To create a standard solution, ethanol was mixed with 0-500 g/mL of quercetin [29].
Scanning Electron Microscopy (SEM): Using a scanning electron microscope, Chia seedstreated with AgNps were examined for structure and surface morphology (JEOL, Musashino, Tokyo Metropolitan, Japan).
FTIR (Fourier Transform Infrared): Grinded Chia seed samples were pressed by the ATR accessories straight onto the diamond ATR crystal. All seed Samples attenuated total reflectance spectra were scanned with a resolution of 4 cm−1, and 4 scans were accumulated per spectrum. All spectra were recorded in triplicate, and the average of three measurements was calculated for each. Prior to each ATR experiment, an air background spectrum was acquired. Spectral data was collected by compressing the sample with the ATR accessory. The ATR cell was cleaned with ethyl alcohol and warm water.
Statistical analysis: All analyses were performed in triplicate. The physiocchemical, nutritional, and antioxidant profile of sprouted Chia seeds that had been Microwaved, germinated, and treated with AgNPs were studied statistically using Analysis of Variance (ANOVA) methods and Statistic 16.0 software. The difference was considered significant if p<0.05.
Table 1: Effect of Silver nanoparticles on proximate composi- tion of germinated and microwaved chia seeds: aAll values are mean of triplicate determinations. Means within a column with different superscripts are significantly different at P < 0.05.
| Samples | Moisture Content (%) | Ash (%) | Fat (%) | Protein (%) | Crude Fiber (%) | Carbohydrate (%) |
|---|---|---|---|---|---|---|
| Raw | 6.64±0.21b | 4.64±0.35b | 31.58±0.98b | 19.94±0.23a | 19.98±1.65a | 17.19±1.37b |
| Go | 12.77±0.71c | 4.32±0.01a | 31.28±0.75a | 21.23±0.91c | 20.24±0.34a | 10.13±1.20a |
| G | 13.23±0.84c | 5.37±0.01c | 31.11±1.08a | 21.13±0.28bc | 19.88±0.34a | 9.26±0.77a |
| Mo | 5.19±0.76a | 6.32±0.01d | 32.19±1.61a | 20.12±0.28ab | 20.52±0.72a | 15.65±0.86b |
| M | 5.85±0.21ab | 4.47±0.11ab | 33.12±0.89a | 19.98±0.73a | 21.13±0.83a | 15.43±2.08b |
Abbreviations: Raw: Raw chia seeds flour; Go: Germinated chia seeds flour; G: 60ppm AgNp germinated chia seeds flour; Mo: micro- waved chia seeds flour; M: 60ppm AgNp microwaved chia seeds flour.
Table 2: Effect of Silver nanoparticles on Total phenols and Fla- vonoids of germinated and microwaved chia seeds: aAll values are mean of triplicate determinations. Means within a column with different superscripts are significantly different at P<0.05.
| Samples |
Total phenols (mg Gallic acid/g) |
Flavonoids (mg Catechin/100g) |
DPPH (%) |
|---|---|---|---|
| Raw | 2.41±0.49b | 1.01±5.39cc | 54.82±0.07b |
| Go | 4.21±0.77d | 1.28±0.54d | 89.37c0.65d |
| G | 5.41±0.41e | 1.30±0.53e | 94.56±1.68e |
| Mo | 1.32±1.81a | 4.41±0.61a | 23.23±0.14a |
| M | 2.82±0.49c | 8.22±0.83b | 64.37±0.37c |
Result and discussion
Effect of silver nanoparticles on germination percentage and root length of chia seeds
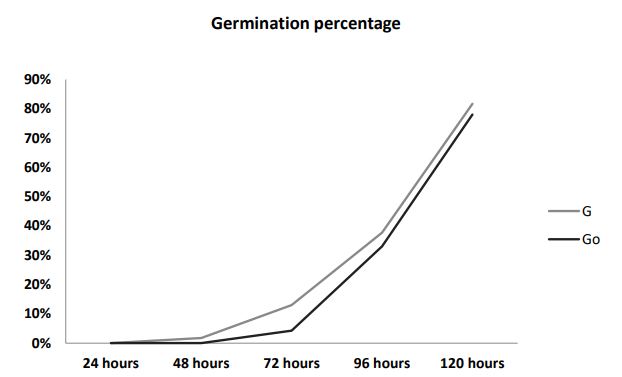
Germination percentage: As shown in Figure 1a, the percentage of control Germination (Go) and germination with 60 ppm AgNP of chia seeds (G) remained zero percent after 24 hours. However, after 48 hours germination started on the seeds treated with AgNPs as compared to the germinated samples without AgNPs (G0) 1.73% and zero percent respectively. After 72 hours, percentage germination started to increase up to 4.26% in Go and 12.76% in G. Subsequently, after 120 hours, percentage germination reached up to 78.00% and 81.66% in Go and G respectively. The seed development process was directly affected by silver nanoparticles as these nanoparticles improved seed germination with less time. This study is parallel to [30] who reported that silver nanoparticles enhanced germination process, plant growth with improvement in photosynthetic quantum efficiency and as antimicrobial agents to manage plant diseases.
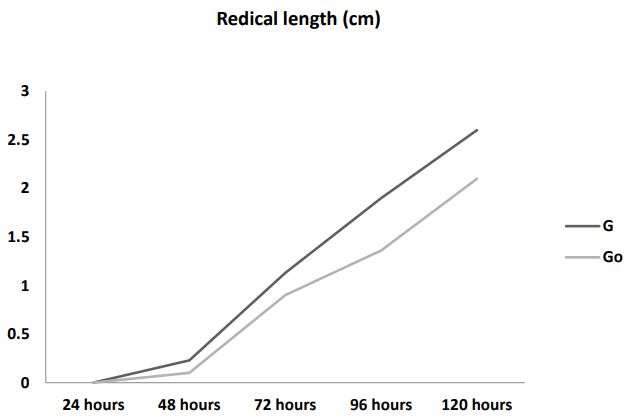
Radicle length: Figure 1b shows the radicle length of germinated chia seeds. Go and G had an average length of 2.1 cm and 2.6 cm respectively. Higher radicle length was observed in seeds germinated with AgNPs as compared to seeds without AgNPs.
Effect of silver nanoparticles on germination and microwaved treatment on proximate composition of chia seeds: The proximate analysis of chia seeds treated with AgNPs followed by germination or microwaved were given in Table 1. Moisture content varied notably across treatments. Germination of chia seeds without AgNPs (Go) and Germination of chia seeds with 60ppm AgNPs (G) samples exhibited the highest moisture levels emphasizing the hydrating effect of germination while microwaved samples (Mo and M) showed lower moisture contents. The wide range in moisture content indicated the influence of treatment methods on water retention in chia seeds. Protein content displays subtle differences with values ranging from 19.94% to 21.23%. Ash content indicative of mineral composition ranges from 4.32% to 6.32%. Germinated samples Go showed slight decrease in ash content compared to raw seeds while microwave-treated samples (Mo and M) exhibited variable ash levels. The observed differences underscore the influence of treatments on the mineral composition of chia seeds. Total fat content ranges from 31.11% to 33.12% as shown in Table 1. All samples showed insignificant differences. It means that there is no effect in fat content while giving different treatments to chia seeds. Germinated samples (Go and G) exhibited slightly higher protein levels compared to raw seeds, suggesting a potential enhancement of protein content during germination. Microwave treatments (Mo and M) show protein content within a similar range of raw seeds, indicating the resilience of protein levels to these treatments. Carbohydrate content ranges from 9.26% to 17.19%, with the lowest value in Germinated control (Go) and the highest in raw seeds. This variation suggests that germination leads to a reduction in carbohydrate content. Microwave treatments show intermediate values, indicating a moderate impact on carbohydrate composition. Crude fiber content ranges from 19.88% to 21.13%, with the highest value in microwaved sample M and the lowest in Germinated control (Go). This variation implies that microwave treatment may contribute to a slight increase in crude fiber content compared to germination. In conclusion, the comparison between high and low values highlights the diverse effects of germination and microwave treatments on the proximate composition of chia seeds. These findings contribute valuable insights into the nutritional and functional properties of chia seeds under different processing conditions, offering potential applications in food and nutrition.
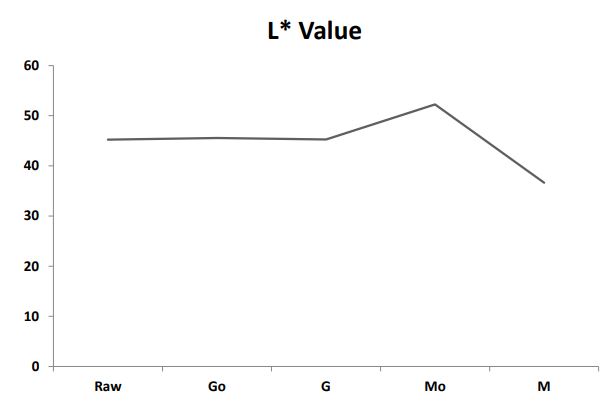
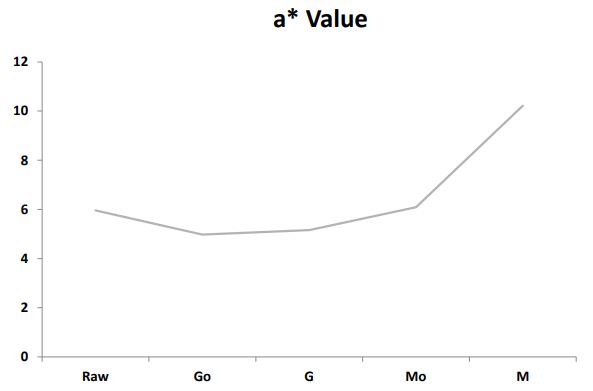
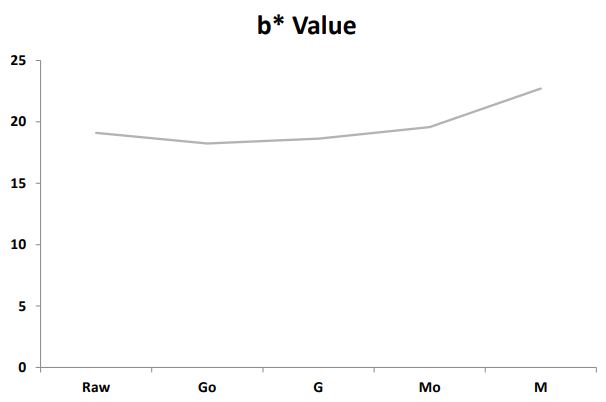
Effect of silver nanoparticles on germination and microwaved treatment on color profile of chia seeds: The effect of 60 ppm silver nanoparticles on germination and microwaved treatment on Color Profile of chia seeds is shown in Figure 2a2c. The comparison of L* a* b* values among the samples revealed notable variations in their color characteristics. In terms of Lightness (L*) as shown in Figure 2a, sample M demonstrated a significant decrease with a value of 36.65 distinctly lower than Raw, Go, G, and Mo. Regarding (a*) as shown in Figure 2b M exhibited a substantially higher value of 10.22 compared to the other samples indicating a pronounced shift towards unusual tones. Additionally, in (b*) as shown in Figure 2c, M stands out with a markedly higher value of 22.71, suggesting a distinctive shift towards different tones in comparison to Raw, Go, G, and Mo. These numerical changes in L* a* b* values emphasize the unique color characteristics introduced by the microwaving process with silver nanoparticles in sample M, setting it apart from the other samples in terms of lightness and color tones.
The effect of silver nanoparticles on bioactive compound of germinated and microwaved chia seeds: The examination of total phenols in various samples revealed distinct concentrations, providing insights into their phenolic composition. G exhibited the highest concentration (5.41±0.41 mg Gallic acid/g) presented in Table 2, suggesting potential applications or implications. Conversely, Mo displayed the lowest concentration (1.32±1.81 mg Gallic acid/g), warranting further investigation into factors influencing its phenolic content. Comparison of Raw, Go, and M samples emphasized the impact of processing on phenolic levels. Table 2 showed that raw (2.41±0.49 mg Gallic acid/g) had a lower concentration than Go (4.21±0.77 mg Gallic acid/g) and M (2.82±0.49 mg Gallic acid/g), indicating the influence of treatment methods. The standard deviations highlight result variability, necessitating exploration of potential sources, such as sample heterogeneity or experimental procedures. Mo stands out with the highest flavonoid content, recording a mean value of 4.41±0.61 mg Catechin/100 g. This finding suggests a potentially unique composition in Mo, and further exploration into the factors contributing to this elevated flavonoid level could provide valuable insights. Similarly, M exhibits a substantial flavonoid concentration with a mean value of 8.22±0.83 mg Catechin/100 g. The markedly higher content in M compared to other samples may have implications for insert potential applications or significance. Conversely, Raw, Go, and G display lower mean flavonoid values of 1.01±5.39, 1.28±0.54, and 1.30±0.53 mg Catechin/100 g, respectively. The processing methods, represented by Go and G, appear to have a limited impact on flavonoid levels compared to the raw sample. This prompts further investigation into the specific processing methods and their effects on flavonoid retention or alteration. The assessment of antioxidant activity, measured by DPPH scavenging capacity (mg Catechin/100 g), reveals distinct levels across Raw, Go, G, Mo, and M samples in Table 3. G stands out with the highest DPPH scavenging activity (94.56±1.68 mg Catechin/100 g), indicating significant antioxidant potential. This finding emphasizes the potential health benefits associated with G, potentially attributed to specific compounds or processing methods. M also demonstrates notable antioxidant activity (64.37±0.37 mg Catechin/100 g), suggesting potential health implications. Further exploration into the specific compounds contributing to antioxidant activity in M could enhance our understanding of its health-related properties. Conversely, Raw, Go, and Mo exhibit varying levels of DPPH scavenging activity 54.82±0.07, 89.37±0.65, and 23.23±0.14 mg Catechin/100 g respectively. These differences prompt considerations of factors such as processing methods or inherent properties of the raw materials influencing antioxidant potential. Comparisons with existing literature would provide context and insights into how these samples fare in terms of antioxidant activity. Understanding these variations contributes to a broader perspective on the potential health benefits associated with their consumption. The diverse antioxidant activities observed in the samples underscore their potential in promoting health and well-being. Further investigations into specific compounds contributing to these activities would enhance our understanding and could reveal valuable applications for these samples.
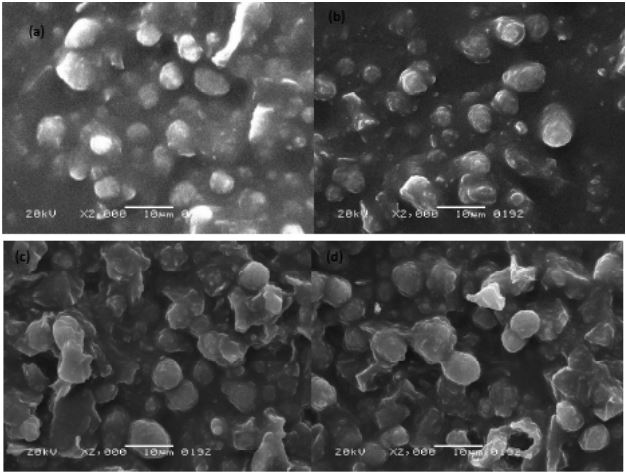
Effect of silver nanoparticles on germination and microwaved treatment on morphological study of chia seeds: The SEM results revealed distinct morphological changes in the samples subjected to various treatments, shedding light on the structural alterations induced by microwaving, germination, and the introduction of silver nanoparticles. The microwaved samples (M and Mo) as shown in Figure 3a and 3b exhibited a noticeable shrinkage in comparison to their counterparts. This shrinkage may be attributed to the application of microwave energy which could lead to changes in the internal structure of the samples. The observed reduction in size suggested a significant impact on the overall potentially influenced by heat-induced alterations and the specific interactions of microwaves with the sample components. Conversely, the germinated samples (G and Go) as shown in Figure 3c and 3d displayed an expansion in their structures. The germination process driven by enzymatic activity is known to induce changes in the composition of seeds. This expansion is indicative of the dynamic cellular processes associated with germination such as cell elongation and increased water uptake. The expansion in these samples suggested that germination contributes to structural changes potentially enhancing certain characteristics. The addition of silver nanoparticles in M and G introduces another dimension to the observed structural variations. The incorporation of silver nanoparticles in both samples may have additional effects on the microstructure. The impact of silver nanoparticles on the samples could involve interactions at the nanoscale influencing the overall morphology and potentially contributing to unique features not observed in the microwaved or germinated samples alone. SEM results highlighted the distinct structural responses of the samples to different treatments. The observed shrinkage in microwaved samples and expansion in germinated samples combined with the influence of silver nanoparticles provided valuable insights into the intricate interplay between treatments and structural modifications. These findings contributed to our understanding of how microwaving, germination, and nanoparticle incorporation influence the microstructure of the samples paving the way for further exploration and potential applications in various fields.
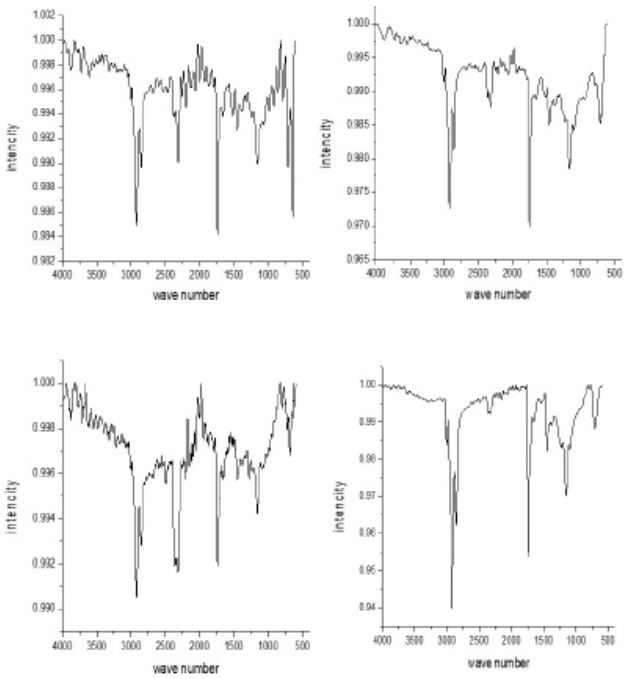
Effect of silver nanoparticles on germination and microwaved treatment on FTIR analysis of chia seeds: The FTIR analysis of chia seeds is shown in Figure 4. Microwaved chia seeds including those treated with silver nanoparticles revealed intricate changes in seed composition induced by both microwaving and silver nanoparticle exposure. In microwaved seeds, N-H stretching at 2925 cm-1 suggested amine salt presence possibly from ammonium ions. The 3000-2800 cm-1 range indicated C-H stretching (alkane) revealing alterations in the lipid or hydrocarbon profile. Peaks at 2360 and 2311 cm-1 suggest O=C=O stretching possibly linked to carbon dioxide release during microwaving. The 1750-1735 cm-1 range corresponded to C=O stretching (carboxylic acid), suggesting changes in carboxylic acid presence. The 1165 cm-1 peak indicates C-O stretching (aliphatic ether), revealing modifications in ether-containing compounds. The 1210-1163 cm-1 range implied C-O stretching (ester) suggesting ester presence from carboxylic acids and alcohols. Peaks at 712 and 641 cm-1 correspond to C=C bending (alkene), indicating unsaturated hydrocarbons. Silver nanoparticle treatment introduces notable changes, such as O-H stretching (3012 cm-1) and continued C-H stretching (2920, 2849 cm-1). The 2318 cm-1 peak suggests O=C=O stretching, potentially influenced by silver nanoparticles. Peaks at 1738 cm-1 and 1455 cm-1 reveal C-H bending and CH3 groups indicating alterations in aromatic compounds and lipids/proteins. Peaks at 1148 and 1158 cm-1 suggest ester presence indicating chemical modifications due to silver nanoparticle treatment. The 705 cm-1 peak corresponds to C=C bending (alkene), showing continued unsaturated hydrocarbons. In control germinated chia seeds (Go), functional groups are evident, such as O-H stretching (3614 cm-1) and N-H stretching (3394, 3309 cm-1). The 3217 cm-1 peak indicates O-H stretching (carboxylic acid) and the 3000-2840 cm-1 range shows C-H stretching (alkane). Peaks at 2360 and 2326 cm-1 suggest O=C=O stretching, and 1745 cm-1 indicates C=O stretching (carboxylic acid). The 1400-1460 cm-1 range and 1158 cm-1 peak reveal CH3 groups and C-O stretching (ester), respectively. The 683 cm-1 peak corresponds to C=C bending (alkene), indicating unsaturated hydrocarbons. Silver nanoparticle-germinated chia seeds exhibit differences, including O-H stretching (3012, 3005 cm-1) and continued C-H stretching (2920, 2849 cm-1). Peaks at 2333 and 2162 cm-1 suggest O=C=O stretching, and 1653 cm-1 reveals C=C stretching (alkene). The 1158 cm-1 peak indicates C-O stretching (ester), and the 712 cm-1 peak corresponds to C=C bending (alkene). This comparison emphasizes chemical distinctions induced by silver nanoparticle treatment.
Conclusion
This study delved into the intricate interactions between silver nanoparticles (AgNPs) and chia seeds (Salvia Hispanica L.) throughout the germination process and subsequent microwave treatment. The investigation explored the effects of AgNPs on chia seed germination, as well as the impact of microwave treatment on chia seeds treated with AgNPs. The following key findings emerged from the study:
Germination and growth: The application of AgNPs at a concentration of 60 ppm significantly influenced the germination percentage and radicle length of chia seeds. The results indicated that silver nanoparticles enhanced seed germination, with the highest percentage achieved at 81.67% on the fifth day. Additionally, the radicle length increased with AgNP treatment suggesting a positive impact on early seedling growth.
Proximate composition: The study revealed significant variations in the proximate composition of chia seeds subjected to different treatments. Germinated samples showed higher moisture and protein content, while microwave-treated samples exhibited increased fat content. These variations provide insights into the nutritional implications of different processing methods.
Color profile: Microwave treatment with AgNPs induced unique color characteristics in chia seeds, as evidenced by changes in Lab* values. Sample M exhibited decreased Lightness (L*) and increased values for the color parameters (a* and b*), indicating a distinctive shift in color tones.
Microstructural changes: Scanning Electron Microscopy (SEM) analysis highlighted distinct morphological changes in chia seeds subjected to various treatments. Microwave treatment resulted in noticeable shrinkage, while germinated samples displayed structural expansion. The addition of AgNPs introduced additional complexity to the microstructure.
Bioactive component and antioxidant activity: The study investigated the influence of AgNPs on bioactive compounds, including total phenols and flavonoids, as well as antioxidant activity. Germinated samples exhibited higher concentrations of total phenols, flavonoids, and DPPH scavenging activity, suggesting potential health benefits associated with these samples. In summary, the findings underscore the multifaceted effects of AgNPs, germination, and microwave treatment on the germination, composition, color, microstructure, and bioactivity of chia seeds. These insights contribute to the growing body of knowledge on the application of nanotechnology in agriculture and food processing. The study’s comprehensive approach provides a foundation for future research and potential applications in improving the nutritional and functional properties of chia seeds in diverse fields, from agriculture to food science and beyond.
References
- Isigonis P, Afantitis A, Antunes D, Bartonova A, Beitollahi A, et al. Risk governance of emerging technologies demonstrated in terms of its applicability to nanomaterials. Small. 2020; 16(36): 2003303.
- Pramanik P, Krishnan P, Maity A, Mridha N, Mukherjee A, et al. Application of nanotechnology in agriculture. Environmental Nanotechnology. 2020; 4: 317-348.
- Kim D Y, Kadam A, Shinde S, Saratale R G, Patra J, et al. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. Journal of the Science of Food and Agriculture. 2018; 98(3): 849-864.
- Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. International Journal of Molecular Sciences. 2021; 22(13): 7202.
- Alabdallah N M, Hasan M M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi Journal of Biological Sciences. 2021; 28(10): 5631-5639.
- Ali N M, Yeap S K, Ho W Y, Beh B K, Tan S W, et al. The promising future of chia, Salvia Hispanica L. Journal of Biomedicine and Biotechnology. 2012.
- Barazandegan Y. Extraction, optimization, and characterization of chia seed mucilage as an emerging biopolymer (Doctoral dissertation, University of Missouri-Columbia). 2023.
- Kulczyński B, Kobus-Cisowska J, Taczanowski M, Kmiecik D, Gramza-Michałowska A. The chemical composition and nutritional value of chia seeds-Current state of knowledge. Nutrients. 2019; 11(6): 1242.
- Orona-Tamayo D, Valverde M E, Paredes-López O. Bioactive peptides from selected Latin American food crops-A nutraceutical and molecular approach. Critical reviews in food science and nutrition. 2019; 59(12): 1949-1975.
- Miyahira R F, Lopes J D O, Antunes A E C. The use of sprouts to improve the nutritional value of food products: A brief review. Plant Foods for Human Nutrition. 2021; 76(2): 143-152.
- Ikram A, Saeed F, Afzaal M, Imran A, Niaz B, et al. Nutritional and end‐use perspectives of sprouted grains: A comprehensive review. Food science & nutrition. 2021; 9(8): 4617-4628.
- Maia Y L M, de Sousa Correia M L, Melo F L D. Saúde e sustentabilidade em grãos: Germinados, brotos e microgreens. Referências em Saúde do Centro Universitário Estácio de Goiás. 2020; 3(02): 147-157.
- European Commission. Commission Implementing Regulation (EU) No 208/2013. OJEU. Https://eur-lex.europa.eu/eli/reg_impl/2013/208/oj. Accessed 11 April 2022. 2013.
- Pająk P, Socha R, Broniek J, Królikowska K, Fortuna T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food chemistry. 2019; 275: 69-76.
- Abdel-Aty A M, Elsayed A M, Salah H A, Bassuiny R I, Mohamed S A. Egyptian chia seeds (Salvia Hispanica L.) During germination: Upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Science and Biotechnology. 2021; 30: 723-734.
- Jan K, Ahmad M, Rehman S, Gani A, Khaqan K. Effect of roasting on physicochemical and antioxidant properties of kalonji (Nigella sativa) seed flour. Journal of Food Measurement and Characterization. 2019; 13: 1364-1372.
- Potočnik T, Cizej M R, Košir I J. Influence of seed roasting on pumpkin seed oil tocopherols, phenolics and antiradical activity. Journal of Food Composition and Analysis. 2018; 69: 7-12.
- Hatamian M, Noshad M, Abdanan-Mehdizadeh S, Barzegar H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS Journal. 2020; 21: 1-8.
- Jogihalli P, Singh L, Sharanagat V S. Effect of microwave roasting parameters on functional and antioxidant properties of chickpea (Cicer arietinum). LWT-Food Science and Technology. 2017; 79: 223-233.
- Santos J, Alvarez‐Ortí M, Sena‐Moreno E, Rabadán A, Pardo J E et al. Effect of roasting conditions on the composition and antioxidant properties of defatted walnut flour. Journal of the Science of Food and Agriculture. 2018; 98(5): 1813-1820.
- Cerretani L, Bendini A, Rodriguez-Estrada M T, Vittadini E, Chiavaro E. Microwave heating of different commercial categories of olive oil: Part I. Effect on chemical oxidative stability indices and phenolic compounds. Food Chemistry. 2009; 115(4): 1381-1388.
- Yasur J, Rani P U. Environmental effects of nano silver: Impact on castor seed germination, seedling growth, and plant physiology. Environmental Science and Pollution Research. 2013; 20: 8636-8648.
- Özcan M M, Al-Juhaimi F Y, Ahmed I a M, Osman M A, Gassem M A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chemistry. 2019; 278: 190-196.
- Latimer Jr. Official methods of analysis (Moisture-Protocol No. 925.10, Crude Ash Protocol No. 923.03, Fat-Protocol No. 920.85, Fiber - Protocol No. 32-10, and Protein-Protocol No. 920.87) (19th edn.). Association of Official Analytical Chemists (AOAC). 2019.
- Yi J, Kebede B T, Doan N H D, Buve C, Grauwet T, et al. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT-Food Science and Technology. 2017; 75: 85-92.
- Sultana B, Anwar F, Iqbal S. Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. International Journal of Food Science & Technology. 2008; 43(3): 560-567.
- Mugwagwa L R, Chimphango A F A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydrate polymers. 2019; 219: 29-38.
- Lee K W, Kim Y J, Lee H J, Lee C Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. Journal of agricultural and food chemistry. 2003; 51(25): 7292-7295.
- Aryal S, Baniya M K, Danekhu K, Kunwar P, Gurung R, et al . Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019; 8(4): 96.
- Almutairi ZM, Alharbi A. Effect of silver nanoparticles on seed germination of crop plants. International Journal of Nuclear and Quantum Engineering. 2015; 9(6): 689-93.