SciBase Journals
SciBase Gastroenterology and Hepatology
- Article Type: Review Article
- Volume 1, Issue 1
- Received: May 23, 2024
- Accepted: Jun 26, 2024
- Published Online: Jul 03, 2024
Early Diagnosis of Cancer of the Pancreas: A Key Step in Improving Survival Rates
Stephen Malnick*; Ali Abdullah; Sorcha Mildiner
Department of Internal Medicine C, Kaplan Medical Center, Rehovot 76100, Israel (Affiliated to the Hebrew University, Jerusalem, Israel).
*Corresponding Author: Stephen Malnick
Department of Internal Medicine C, Kaplan Medical Center, Rehovot 76100, Israel (Affiliated to the Hebrew University, Jerusalem, Israel).
Email: steve_m@clalit.org.il
Abstract
Abbreviations: AUC: Area Under The Curve; ENDPAC: Enriching New Onset Diabetes For Pancreatic Cancer; EUS: Endoscopic Ultrasound; IPMN: Intraductal Papillary Mucinous Neoplasm; MRCP: Magnetic Resonance Cholangiopancreatoraphy; MRI: Magnetic Resonance Imaging; NOD: New Onset Diabetes; PDAC: Pancreatic Duct Adenocarcinoma.
Citation:Malnick S, Abdullah A, Mildiner S. Early Diagnosis of Cancer of the Pancreas: A Key Step in Improving Survival Rates. SciBase Gastroenterol Hepatol. 2024; 1(1): 1001.
Introduction
Cancer of the pancreas has a lifetime incidence in the United States of 1.6% [1]. It accounts for 3.2% of all new cancers and is the 12th most common cancer. The survival of those diagnosed with cancer of the pancreas is unfortunately poor. The majority of pancreatic carcinoma patients are diagnosed at advanced stages of disease [2]. Only 15-20% of patients are candidates for surgical resection at diagnosis and the 5 year survival is less than 10% [3,4]. It is expected to become the second leading cause of death from cancer within a decade [5]. Cancer of the pancreas is the only common cancer for which early detection is not universally available.
The natural history of development of pancreatic duct carcinoma is a progression from carcinoma in situ, to minute lesions less than 10 mm in diameter, through to small lesions less than 20 mm in diameter and large resectable lesions of approximately 30 mm in diameter. The lesions that are less than or equal to 20mm in diameter have a five year survival over 85% and are potentially curable.
Early detection of cancer of the pancreas
There are several barriers to the detection of early sporadic Pancreatic Duct Adenocarcinoma (PDCA). Firstly, it is an uncommon disease with an incidence in those aged more than 50 years of age of approximately 38 per 100,000 people [1]. Screening will therefore need to be restricted to those groups with a high risk of a high incidence of PDAC. By the time patients have symptoms they most likely have advanced disease and thus screening will need to be concentrated on those individuals who are asymptomatic or minimally symptomatic.
Screening is not recommended for average risk individuals , but only for those patients with an increased risk due to genetic susceptibility [6-12]. PDAC progresses rapidly, and thus there is a limited time period available for early detection [13].
There is a benefit to early detection of PDCA. For those at high risk who undergo screening between 60 to 70% will have an early stage cancer with almost 705 chance of 5 year survival and a mean overall survival of 10 years [14].
Risk factors
The risk factors for pancreatic carcinoma include a family history, germline mutations, pancreatic cysts, chronic pancreatitis obesity, smoking age, race and blood group.
A positive family history is defined by presence of the disease in 2 or more first-degree relatives. The risk increases with the number of first degree relatives affected. For those with 2 first degree relatives the risk is 8-12% and this risk increaser to 40% with 3 first degree relatives [15].
Germline mutations are present in 5.5% of all pancreatic cancers. These are present in 7.9% of those patients with a positive family history and 5.2% of patients without a family history [16]. A high-risk individual is a first-degree relative of a patient with pancreatic carcinoma and more than 2 affected genetically related relatives. In addition, irrespective of a family history of pancreatic cancer, patients with Peutz-Jeghers syndrome, or PRSS1 with hereditary pancreatitis or the CDKN2A mutation are classified as high-risk. In addition patients with Lynch syndrome and at least one first degree relative with pancreatic cancer or patients with a germline variant in BRCA, PALB 2 and ATM genes together with one first degree relative with PDCA are also individuals at high risk for developing pancreatic cancer [10].
Methods for early detection
Screening in most high-risk patients should start at the age of 50 or 10 years earlier than the earliest case of pancreatic cancer known in the family. Certain subgroups at very high risk need to be screened earlier. Those with Peutz-Jeghers syndrome (STK11/LKB1) at age of 35, those with hereditary pancreatitis (PRSS1) at age 40. In addition, patients with familial atypical multiple mole melanoma syndrome (CDKN2A) also need to be screened at the age of 40.
Conventional axial imaging is not sensitive enough for early detection of PDAC [17]. High resolution CT scan appeared to be more promising [18] but is still not recommended in guidelines. Retrospective studies of CT scans performed for other indications show no evidence of stage 4 disease 6 months before the diagnosis of PDAC was made, which supports the benefit of early detection [17].
MRI and Endoscopic Ultrasound (EUS) are the most commonly used imaging modalities. The most commonly used diagnostic imaging tests are either MRI or EUS, which are more sensitive for detecting small cystic lesions as compared to CT scanning. MRCP provides a better determination of cyst communication with the main pancreatic duct [19] MRI may have less sensitivity in detecting small solid lesions as compared to EUS [20].
Furura et al. [21] have recently published a case of high grade pancreatic intraepithelial neoplasia diagnosed by MRCP. This suggests that there maybe an increase in the sensitivity of MRI with newer machines and more experience.
Strategy for screening high risk patients
Screening for carcinoma of the pancreas by the use of biomarkers such as CA 19-9 is at present insensitive. One study of 546 patients with at least one affected relative found elevated CA19-9 in 4.9%. Of these a neoplastic or malignant lesion was detected on EUS in only 5 patients (0.9%) and only one was a pancreatic adenocarcinoma [22]. Despite this, there is still a recommendation in International Guidelines for Ca19-9 determination [7,23]. Axial imaging by means of CT is insensitive for the small sized PDAC. There is a need for invasive testing such as Endoscopic Ultrasonography (EUS) in order to assist in making the diagnosis.
Pancreatic cysts seen on imaging are a common incidentaloma. A study of 20,515 patients aged more than 50 found an IPMN in 231 (10.9%) [24]. There are International Guidelines for surveillance of pancreatic cysts- the most recent are of the AGA for neoplastic pancreatic cysts and from Kyoto in Japan for the management of IPMNs (intraductal papillary neoplasm of the biliary tract). Branch-duct IPMNs with no high-risk features such as size greater than 3 cm, a dilated main pancreatic duct, a mural nodule or a solid mass) may stop having surveillance if there is no change after 5 years.
There is also an increased risk of pancreatic cancer in chronic pancreatitis [25]. The risk is increased by 22.6 times and the risk in hereditary pancreatitis is increased by 63.4. For those patients with P/LP germline variants in PRSS1 or other hereditary pancreatic genes and a clinical phenotype consistent with pancreatitis, screening should start 20 years after the onset of pancreatitis or at age 40- whichever is earlier. Screening is not recommended currently in cases not associated with hereditary pancreatitis.
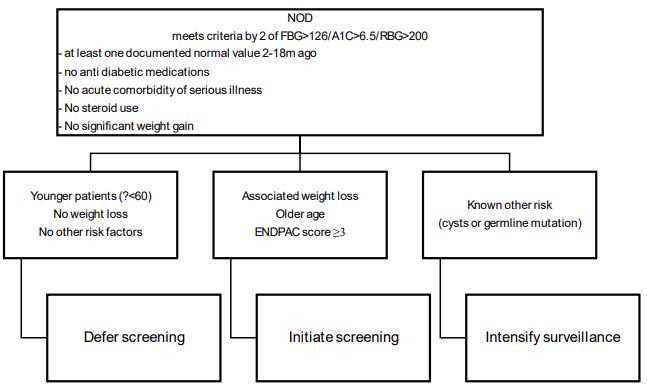
New Onset, Diabetes (NOD), after the age of 50 results in a 6 to 8 times increased risk of PAD within three years [26]. About 20% of patients with PD will also have NOD and approximately one percent of the patients with NOD will have a diagnosis of PDC made in the subsequent three years [18]. Upon diagnosis of PDAC impaired glucose tolerance is present in about 75% of patients [26-30]. In the Nurses Health Study and a health professionals follow-up study over 30 years there were 112,818 females and 46,207 men. 1116 cases of pancreatic cancer were diagnosed. New-onset diabetes mellitus (HR 2.97), in the presence of weight loss greater than 4 kg (HR 6.75) resulted in a marked increase in the risk for developing PDCA. Older age, a previously healthy weight and no intended weight loss increased the risk further [31]. In addition, diabetes mellitus is is more prevalent in PDAC compared to controls (47% vs 7%) [27].
This indicates it may be possible to detect PDA earlier in patients with NOD. The Enriching New- onset Diabetes for Pancreatic Cancer (ENDPAC) score stratified those patients with NOD into those at very low risk, intermediate risk, and very high risk for developing PDAC [26].
The ENDPAC model has recently been validated in a cohort of 6301 patients with NOD based on HBA1C levels. The Area Under the Curve (AUC) was 0.72 [32] sensitivity and specificity were 80%.
We present in the Figure our approach to screening for early PDCA.
Table 1: Gene mutations associated with an increased risk for carcinoma of the pancreas.
| Genes | Odds Ratio |
|---|---|
| CDKN2A | 12.33 |
| TP53 | 6.70 |
| MLH 1 | 6.66 |
| BRCA2 | 6.2 |
| ATM | 5.71 |
| BRCA1 | 2.58 |
A new model for early detection of PDCA has been proposed. This is based on defining a high-risk group for PDAC, enriching it to further define a very high risk group and to find the malignant lesion in this very high-risk group [33].
The established risk factors for PDAC include the number of first degree relatives with a history of PDAC [23]. The risk is higher in those with at least two first degree relatives. In addition, there are inherited syndromes that predispose to PDCA including hereditary breast and ovarian cancer with BRCA1, BRCA2, PALB2, familial polyposis with APC mutations, hereditary pancreatitis with PRSS1 mutations and the Lynch syndrome [9,34]. There are also germline mutations linked to pancreatic cancer [16]. These include ATM and CDKN2A.
Certain lifestyle choices have been linked with a higher risk for developing PDCA. Cigarette smoking increases the risk for pancreatic cancer [7,8]. The risk increases with the amount of cigarettes consumed [13,14,17]. Excess risk decreases with smoking cessation. In a prospective study, the relative risk of pancreatic cancer among current smokers was 2.5 [18]. The risk fell by 48 percent by two years after discontinuing smoking and leveled off 10 to 15 years after stopping, eventually falling to the level of nonsmokers. It has been estimated that cessation of smoking could eliminate approximately 25 percent of pancreatic cancer deaths in the United States [15,18]. High BMI and physical inactivity are also risk factors for PDAC.
The ability to identify those high-risk individuals will aid in making early diagnosis of PDAC possible. Attention has been turning to liquid biopsy. This refers to assaying cancer-related material from a blood sample for changes in DNA, RNA, proteins and other substances [25]. There are limitations to detection from blood. Serum Ca19-9 is elevated in pancreatitis and cholestasis, and, in addition, between 10 and 20% do not produce this marker. Methylated DNA markers such as cfDNA and CtDNA have a lower sensitivity for early-stage cancers and are limited in detecting high grade dysplasia.
Testing of cyst fluid after aspiration by EUS may assist in determining the cyst subtype. KRAS and GNAS mutations are present in IPMN [35].
The accuracy of this for EUS, CT and MRI imaging may increase through the application of artificial intelligence [36]. There are several reports that a radiomics-based machine learning model can predict pancreatic cancer 3 months to 3 years before the clinical diagnosis can be made [37]. In addition, there is an enhancement for detection of visually occult small pancreatic tumors with a sensitivity of 80% for T1 tumors on contrast CT images [38].
Conclusion
Cancer of the pancreas has a historically very poor prognosis. Current efforts are concentrated on identifying those patients at high risk for the disease in order to develop a cost-effective screening program. This is based on family history, lifestyle risk factors, genetic mutations and metabolic disorders especially new-onset diabetes and insulin resistance. We discuss this strategy and the central importance of diabetes in aiding the diagnosis.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020; 70(1): 7-30.
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003; 237(1): 74-85.
- Sun H, Ma H, Hong G, Sun H, Wang J. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database. 1981-2010. Sci Rep. 2014; 4: 6747.
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet (London, England). 2016; 388(10039): 73-85.
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014; 74(11): 2913-21.
- Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2019; 322(5): 438-44.
- Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020; 69(1): 7-17.
- Sawhney MS, Calderwood AH, Thosani NC, Rebbeck TR, Wani S, et al. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Summary and recommendations. Gastrointest Endosc. 2022; 95(5): 817-26.
- Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015; 110(2): 223-62; quiz 263.
- Aslanian HR, Lee JH, Canto MI. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology. 2020; 159(1): 358-62.
- Săftoiu A, Hassan C, Areia M, Bhutani MS, Bisschops R, et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020; 52(4): 293-304.
- Daly MB, Pal T, Berry MP, Buys SS, Dickson P, et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021; 19(1): 77-102.
- Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology. 2018; 155(2): 490-500.e2.
- Dbouk M, Katona BW, Brand RE, Chak A, Syngal S, et al. The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival. J Clin Oncol. 2022; 4(28).
- Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology. 2010; 139(4): 1076-1080.e2. Available from: http://dx.doi.org/10.1053/j.gastro.2010.08.012
- Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018; 319(23): 2401-9.
- Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009; 10(1): 88-95.
- Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019; 156(7): 2024-40.
- Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, et al. Frequent detection of pancreatic lesions in asymptomatic highrisk individuals. Gastroenterology. 2012; 142(4): 795-6.
- Harinck F, Konings ICAW, Kluijt I, Poley JW, Van Hooft JE, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016; 65(9): 1505-13.
- Furuya N, Yamaguchi A, Kato N, Sugata S, Hamada T, et al. Highgrade pancreatic intraepithelial neoplasia diagnosed based on changes in magnetic resonance cholangiopancreatography findings: A case report. World J Clin Cases. 2024; 12(8): 1487-96.
- Zubarik R, Gordon SR, Lidofsky SD, Anderson SR, Pipas JM, et al. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011; 74(1): 87-95.
- Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004; 64(7): 2634-8.
- De la Fuente J, Chatterjee A, Lui J, Nehra AK, Bell MG, et al. LongTerm Outcomes and Risk of Pancreatic Cancer in Intraductal Papillary Mucinous Neoplasms. JAMA Netw Open. 2023; 6(10): E2337799.
- Gandhi S, De La Fuente J, Murad MH, Majumder S. Chronic Pancreatitis Is a Risk Factor for Pancreatic Cancer, and Incidence Increases with Duration of Disease: A Systematic Review and Meta-analysis. Clin Transl Gastroenterol. 2022; 13(3): E00463.
- Sharma A, Kandlakunta H, Nagpal SJS, Feng Z, Hoos W, et al. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology. 2018; 155(3): 730-739.e3.
- Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, et al. Early detection of Andersen DK, Korc M, Petersen GM, Eibl G, Li D, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017; 66(5): 1103-10.
- Pizzato M, Turati F, Rosato V, La Vecchia C. Exploring the link between diabetes and pancreatic cancer. Expert Rev Anticancer Ther. 2019; 19(8): 681-7.
- Sharma S, Tapper WJ, Collins A, Hamady ZZR. Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus. Gastroenterology. 2022; 162(6): 1665-1674.e2.
- Gablo N, Trachtova K, Prochazka V, Hlavsa J, Grolich T, et al. Identification and validation of circulating micrornas as prognostic biomarkers in pancreatic ductal adenocarcinoma patients undergoing surgical resection. J Clin Med. 2020; 9(8): 1-12.
- Khan S, Safarudin RF, Kupec JT. Validation of the ENDPAC model: Identifying new-onset diabetics at risk of pancreatic cancer. Pancreatol Off J Int Assoc Pancreatol. 2021; 21(3): 550-5.
- Maitra A, Sharma A, Brand RE, Van Den Eeden SK, Fisher WE, et al. A Prospective Study to Establish a New-Onset Diabetes Cohort: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018; 47(10): 1244-8.
- Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley J-W, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit Paniccia A, Polanco PM, Boone BA, Wald AI, McGrath K, et al. Prospective, Multi-Institutional, Real-Time NextGeneration Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology. 2023; 164(1): 117-133.e7. Available from: https://doi.org/10.1053/j.gastro.2022.09.028.
- Huang B, Huang H, Zhang S, Zhang D, Shi Q, et al. Artificial intelligence in pancreatic cancer. Theranostics. 2022; 12(16): 6931-54.
- Mukherjee S, Patra A, Khasawneh H, Korfiatis P, Rajamohan N, et al. Radiomics-based Machine-learning Models Can Detect Pancreatic Cancer on Prediagnostic Computed Tomography Scans at a Substantial Lead Time Before Clinical Diagnosis. Gastroenterology. 2022; 163(5): 1435-1446.e3.
- Korfiatis P, Suman G, Patnam NG, Trivedi KH, Karbhari A, et al. Automated Artificial Intelligence Model Trained on a Large Data Set Can Detect Pancreas Cancer on Diagnostic Computed Tomography Scans As Well As Visually Occult Preinvasive Cancer on Prediagnostic Computed Tomography Scans. Gastroenterology. 2023; 165(6): 1533-1546.e4.