SciBase Journals
SciBase Hematology & Blood Disorders
- Article Type: Case Report
- Volume 1, Issue 1
- Received: Jun 20, 2024
- Accepted: Jul 18, 2024
- Published Online: Jul 16, 2024
Mantle Cell Lymphoma- A Rare Occurrence: Case Report
Akshat K Aggarwal; Gurpreet Kaur*; Aneez Ali; Ankur Ahuja; Somasundaram Venkastesan
Department of Pathology, Armed Forces Medical College, Pune-411040, India.
*Corresponding Author: Gurpreet Kaur
Department of Pathology, Armed Forces Medical College, Pune-411040, India.
Email: gurpreet.sagoo37@gmail.com
Abstract
Mantle Cell Lymphoma (MCL) is a relatively uncommon yet distinct type of malignant lymphoma whose clinical and pathological characterization has been limited by the small numbers of cases published to date. It comprises 3-7% of all Hodgkin’s lymphoma cases, with a median diagnosis age of 68 years. Based on immunophenotypic and molecular data, MCL is a neoplasm composed of cells resembling those residing in follicular mantle zones. Although MCL usually presents as advanced disease some patients may present with more limited (stages I-I) disease. We present the case of a 41-year-old male who exhibited generalized lymphadenopathy and hepatosplenomegaly. Peripheral smear showed absolute lymphocytosis and the bone marrow biopsy and flow cytometry identified the condition as mantle cell lymphoma. The patient’s management required a multidisciplinary approach, incorporating haematological, molecular, and genetic evaluations. Treatment included hyper-CVAD chemotherapy. Proteasome inhibitors (Bortezomib), mTOR inhibitors (Temsirolimus), and immunomodulatory drugs (Lenalidomide) have recently been added to the treatment options in MCL.This case underscores the significance of comprehensive evaluation and customized therapeutic strategies and re looking an old diagnosis in managing complex haematological disorders.
Keywords: Non-Hodgkin lymphoma; Mantle cell lymphoma; Cyclin D1; CCND1.
Citation:Aggarwal AK, Kaur G, Ali A, Ahuja A, Venkastesan S. Mantle Cell Lymphoma- A Rare Occurrence: Case Report. SciBase Hematol & Blood Disord. 2024; 1(1): 1001.
Introduction
Mantle Cell Lymphoma (MCL) is a type of mature B-cell nonHodgkin lymphoma characterized by a variable clinical course. MCL possesses unique morphological, immunophenotypic, cytogenetic, and molecular genetic features, some of which have been described on only recently [1]. Cytogenetic and molecular evidence that emerged in the past decade as what was earlier called intermediately differentiated lymphocytic lymphoma, centrocytic lymphoma, and MZL denote a common entity, now referred to as MCL [2]. Here, we present the case of a middleaged male who presented with B symptoms and was diagnosed with MCL.
Case presentation
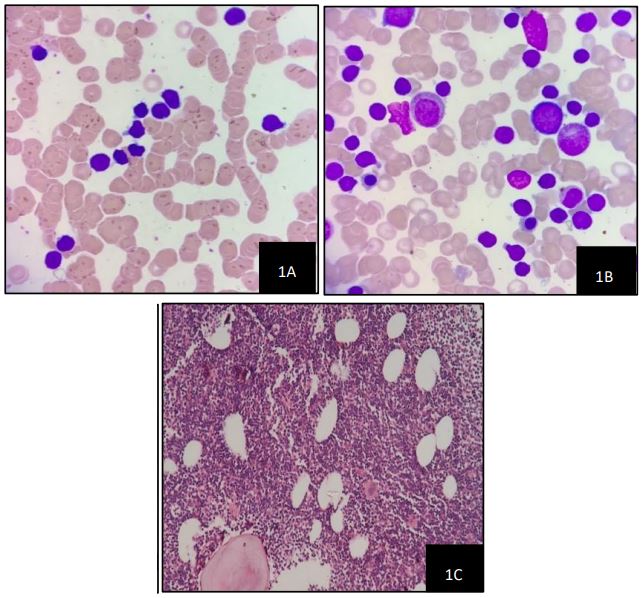
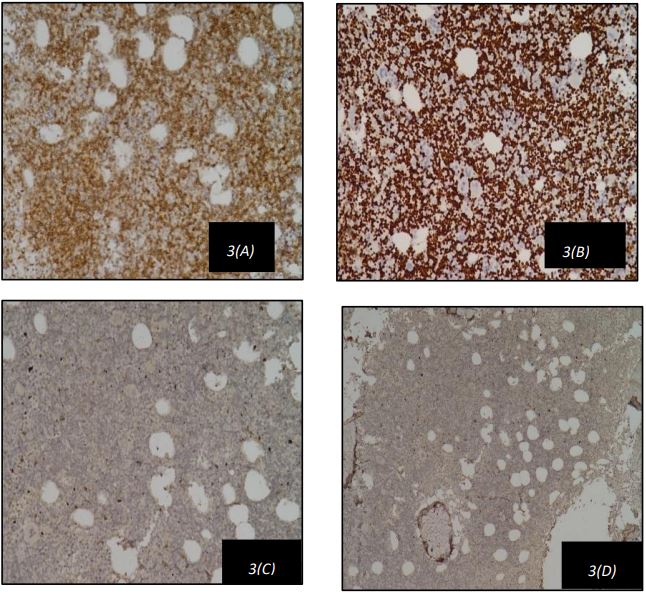
A 42-year-old male, previously diagnosed with a prolapsed intervertebral disk (L5-S1) and irritable bowel syndrome, presented with a three-month history of low-grade fever, malaise, early satiety, and nonspecific musculoskeletal pain. There was no history of weight loss, chronic cough, or bleeding tendencies. Physical examination revealed anaemia, generalized lymphadenopathy, and massive splenomegaly 8 cm below the left costal margin. Laboratory tests showed mild anaemia (haemoglobin: 10.9 g/dL) and leucocytosis (white blood cell count: 116,500/cumm) with lymphocytosis (Absolute Lymphocyte Count: 104,850/cumm). The peripheral blood smear (Figure 1) showed rouleaux formation with presence of atypical lymphocytes which were small to moderate in size, with round nuclei, clumped chromatin. His serum Lactate Dehydrogenase (LDH) was 180 U/L and alkaline phosphatase was 52 U/L. A PET scan indicated massive splenomegaly with diffusely increased uptake in the liver, suggestive of reticuloendothelial system activation. Bone marrow aspirate showed myeloblast,pronormoblast, featuring atypical lymphocytes with intermediate size, high nuclear-to-cytoplasmic ratio, irregular nuclear borders, and finely dispersed chromatin. Figure 2 shows these aspirate findings as described above. Bone marrow biopsy confirmed hypercellular marrow with diffuse lymphoid infiltration as depicted in Figure 2. Immunohistochemistry revealed that these lymphoid cells were positive for CD5 and Cyclin D1 as depicted in Figure 2(A) and Figure 2(D) respectively and negative for CD23 and CD10 as shown in Figure 2(B) and Figure 2(C) respectively.Multiparametric flow cytometry identified atypical cells positive for CD5, CD19, CD23, FMC-7, Kappa, and Lambda, but negative for CD103, CD200, and CD25. Based on these atypical peripheral blood, bone marrow, and immunophenotypic findings, a diagnosis of Mantle Cell Lymphoma (MCL) was made. Further Fluorescence in Situ Hybridization (FISH) testing showed positivity for deletion 13q14.3, trisomy 12, and 14q32 (IGH gene) rearrangement.
The patient commenced treatment with the triangle regimen, R-CHOP chemotherapy (Cyclophosphamide, Doxorubicin, Prednisone, Rituximab, and Vincristine). Haematological parameters and molecular response were closely monitored according to established guidelines. A three-month response assessment showed a significant reduction in lymphocytosis with normalization of leukocyte counts. Regular follow-up visits were scheduled to monitor treatment response and manage adverse effects. Long-term follow-up is essential to evaluate the response to therapy, monitor for disease progression, and manage potential complications.
Discussion
Mantle Cell Lymphoma (MCL) is a B-cell tumour characterized by monomorphic small to medium lymphoid cells with irregular nuclear contours and translocation of the CCND1 gene. MCL accounts for 6% of all non-Hodgkin’s lymphomas in adults, with a median diagnosis age of 60-65 years. Men are 2-6 times more likely to be diagnosed with MCL than women. The median survival time for MCL patients is approximately 3-5 years. The most common “classical” variant of MCL was first described in 1973 by German pathologist K. Lennert [3]. MCL can affect lymph nodes and extra nodal sites such as the gastrointestinal tract, blood, and bone marrow. MCL co-expresses pan-B–cell antigens and CD5 which is a pan-T-cell antigen. A recurring cytogenetic abnormality, t(11;14), occurs in MCL, resulting in rearrangement of the bcl-1 gene locus and overexpression of the cyclin D1 (CCND1/PRAD1) gene. This genetic event is thought to have an important role in the pathogenesis of MCL, because overexpression of cyclin D1 protein is thought to lead to deregulation of the normal cell cycle, particularly at the G1-Sphase transition [4]. It is typically composed of lymphoid cells expressing BCL2, CD5, and nuclear cyclin D1, while lacking CD23 expression. Almost all cases exhibit the t(11;14) chromosomal translocation, which fuses the CCND1 gene (encoding cyclin D1) with the immunoglobulin heavy chain gene, leading to cyclin D1 overexpression. Most MCL patients present with advancedstage disease [5]. The cases that are cyclin D1-negative cases have typical morphology and gene expression profile have been described and often show overexpression of cyclin D2 or D3 [6].
Recently, SOX11 has been described as a diagnostic maker that is equally expressed in D1-positive and D1-negative MCL. MCL is one of the most difficult to treat B-cell lymphomas [7,8]. Although conventional chemotherapy induces highremission rates in previously untreated patients, relapse within a few years is common, contributing to a rather short median survival of 5-7 years [9].
Leukemization of lymphoma, indicating diffuse impairment of hematopoietic organs as the disease progresses, occurs in lymphomas such as follicular lymphoma, marginal zone lymphoma, and MCL [10]. The incidence of bone marrow involvement and leukemization in MCL ranges from 20% to 80%. A crucial pathogenetic stage in MCL development is the translocation of genes encoding cyclin D1 (CCND1) and immunoglobulin heavy chains, t(11;14) (q13; q32). Therefore, the primary diagnostic criteria for MCL include the detection of cyclin D1 through immunohistochemical studies or the identification of t (11;14) (q13; q32) via the D-FISH method [11].
Most MCL cases exhibit an aggressive course, with disseminated involvement of lymphatic and extra nodal organs at diagnosis, corresponding to stage IV of the Ann Arbor classification. This advanced stage is seen in over 70% of MCL patients, as observed in our patient, who presented with intermittent B symptoms since November 2011.
Despite MCL’s initial sensitivity to first-line chemotherapy, subsequent relapses and the development of multidrug resistance led to a poor prognosis. Consolidating the first remission with allogeneic bone marrow or hematopoietic stem cell transplantation may offer a potential cure, though this approach is often limited by the procedure’s toxicity and the patient’s age [12]. The utilization of immunophenotypic and cytogenetic techniques has enhanced the definition of various lymphoproliferative diseases [13]. However, morphological examination remains essential for diagnosis, as it reveals the tumours’ cellular substrate and tissue architecture [14].
Conclusion
This case underscores the importance of a multidisciplinary approach in the diagnosis and management of Mantle Cell Lymphoma. Further improvement of therapy, patient participation may be the limiting factor in well-designed clinical trials. Larger case series to unravel disease heterogeneity, develop predictive markers, and advance therapeutic options. Is required to better understand and treat this disease. Patients with MCL should be encouraged to enroll in clinical trials with a strong translational research program.
Declarations
Conflicts of interest: No.
Funding source: NIL.
Acknowledgments
Images: Akshat K Aggarwal, Aneez Ali.
Conceptualization: Gurpreet Kaur, Ankur Ahuja.
Writing: Akshat K Aggarwal, Gurpreet Kaur
Editing: Gurpreet Kaur
References
- Bosch F, López-Guillermo A, Campo E, Ribera JM, Conde E, Piris MA, et al. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998; 82(3): 567-75.
- Swerdlow SH, Williams ME. From centrocytic to mantle cell lymphoma: A clinicopathologic and molecular review of 3 decades. Hum Pathol. 2002; 33(1): 7-20.
- Podzolkov VI, Vargina TS, Pokrovskaya AE, Safronova TA, Abramova AA. Mantle Cell Lymphoma Case Report. Case Rep Oncol. 2018; 11(3): 814-821.
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997; 89(11): 3909-3918.
- Bernard M, Tsang RW, Le LW, et al. Limited-stage mantle cell lymph- oma: Treatment outcomes at the Princess Margaret Hospital. Leuk Lymphoma. 2013; 54(2): 261-267.
- Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer cell. 2003; 3(2): 185-97.
- Ek S, Dictor M, Jerkeman M, Jirström K, Borrebaeck CA. Nuclear expression of the non-B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood, The Journal of the American Society of Hematology. 2008; 111(2): 800-5.
- Mozos A, Royo C, Hartmann E, De Jong D, Baró C, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. haematologica. 2009; 94(11): 1555.
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009; 27(4): 511-8.
- Podzolkov VI, Vargina TS, Pokrovskaya AE, Safronova TA, Abramova AA. Mantle Cell Lymphoma Case Report. Case Rep Oncol. 2018; 11(3): 814-821.
- Li JY, Gaillard F, Moreau A, Harousseau JL, Laboisse C, et al. Detection of translocation t(11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol. 1999; 154(5): 1449-52.
- Deeg HJ, Sandmaier BM. Who is fit for allogeneic transplantation? Blood. 2010; 116(23): 4762-70.
- Okaly GV, Nargund AR, EV Jayanna PK, Juvva CR, Prabhudesai S. Chronic lymphoproliferative disorders at an Indian tertiary cancer centre - the panel sufficiency in the diagnosis of chronic lymphocytic leukaemia. J Clin Diagn Res. 2013; 7(7): 1366-71.
- Maiques O, Georgouli M, Sanz-Moreno V. Recent advances in tissue imaging for cancer research. F1000Res. 2019; 8:F1000 Faculty Rev-1980.