SciBase Journals
SciBase Neurology
ISSN 2996-3788
- Article Type: Research Article
- Volume 1, Issue 2
- Received: Oct 25, 2023
- Accepted: Dec 12, 2023
- Published Online: Dec 19, 2023
Prognostic Burden of Hemorrhagic Transformation in Acute Ischemic Stroke: A Brief Report from the Real Life
Luca Masotti1*; Elisa Grifoni1; Teresa Sansone1; Mariella Baldini2; Elisabetta Bertini2 ; Sara Giannoni2; Ilaria Di Donato2; Chiara Bini1; Irene Sivieri1; Gina Iandoli1; Marianna Mannini1; Elisa Giglio1 ; Eleonora Brai1; Ira Signorini1; Eleonora Cosentino1; Irene Micheletti1; Elisa Cioni1; Giulia Pelagalli1; Alessandro Dei1; Antonio Giordano1; Francesca Dainelli1; Mario Romagnoli1; Chiara Mattaliano1; Elena Schipani1; Giuseppe Salvatore Murgida1; Stefania Di Martino1; Valentina Francolini1; Paola Bartalucci3
1Internal Medicine II and Stroke Unit, San Giuseppe Hospital, Empoli, Italy.
2Neurology, San Giuseppe Hospital, Empoli, Italy.
3Emergency Department, San Giuseppe Hospital, Empoli, Italy.
*Corresponding Author: Luca Masot
Internal Medicine II and Stroke Unit, San Giuseppe Hospital, Empoli, Italy.
Email: luca.masotti@tin.it
Abstract
Introduction: Hemorrhagic Transformation (HT) represents one of the main complications of acute ischemic stroke, affecting its management and prognosis. Few data exists on prognosis of HT in the real world. Aim of this study was to evaluate the prognostic impact of HT in a real world cohort of patients hospitalized for acute ischemic stroke.
Methods: We retrospectively analyzed the clinical data of patients with acute ischemic stroke consecutively admitted to our Stroke Unit. Patients with HT were compared with those without HT. Modified Rankin Scale (mRS) at hospital discharge and at 90 days were the study outcomes. Poor prognosis was defined as mRS≥4. A multivariate logistic regression analysis was performed to identify independent predictors of outcomes.
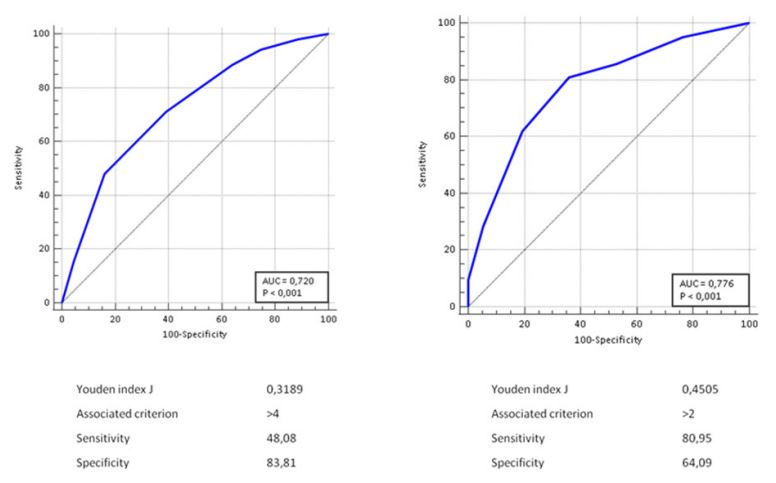
Results: The study population consisted of 564 patients with mean age 77.5±11.8 years. Fifty-two patients (9.2%) showed HT on brain CT. Length of hospitalization, in-hospital mortality, median mRS at discharge, percentage of patients with mRS≥4 at discharge, percentage of HT patients discharged without secondary antiplatelet or anticoagulant prophylaxis and median 90- day mRS were significantly higher in patients with HT than in those without. The presence of HT was predictive of mRS≥4 at discharge and at 90 days (AUC 0.720 and 0.776, respectively). The presence of HT was an independent predictor of poor outcome, increasing the risk by about four times both at discharge (OR 3.64, 95% 1.46-9.06) and at 90 days (OR 4.26, 95% 1.07-16.88).
Conclusions: HT is associated with a poor outcome in terms of mortality and severe residual disability both in the acute phase and at 90 days.
Keywords: Hemorrhagic transformation; Prognosis; Stroke; Mortality; Disability.
Citation: Masotti L, Grifoni E, Sansone T, Baldini M, Bertini E, et al. Prognostic Burden of Hemorrhagic Transformation in Acute Ischemic Stroke: A Brief Report from the Real Life. SciBase Neurol. 2023; 1(2): 1005.
Introduction
Hemorrhagic transformation (HT) is the most feared complication in the hyperacute phase of ischemic stroke, with an incidence of about 5-10% of cases [1,2]. HT can be symptomatic being associated with neurological worsening often leading to coma and death, or more frequently asymptomatic, without significant neurological deterioration [3]. HT has been classified in petechial (type I, isolated petechiae and type II, confluent petechiae) and parenchimal (type I, hematoma less than 30% of ischemic lesion and type II, hematoma major than 30% of ischemic lesion with mass effect). Clinical studies showed that only parenchymal HT, occurring in 3% of ischemic strokes, is associated with a poor outcome [2,4]; on the contrary, petechial HT, occurring in about 5.5% of patients, does not seem to have a negative effect on ischemic stroke [2]. Few data exist about the relationship between stroke patients with HT and short and long term prognosis in real life, therefore the aim of this study was to provide evidence on this topic.
Methods
We retrospectively analyzed clinical data of patients with acute ischemic stroke consecutively admitted to our Stroke Unit along a period two years long. For all patients demographic characteristics (age, sex), pre-stroke modified Rankin Scale (mRS), cardiovascular risk factors, clinical severity using National Institute of Stroke Scale (NIHSS), acute revascularization by systemic thrombolysis and/or mechanical thrombectomy, the site and number of the brain ischemic lesion(s), the presence of large vessel occlusion on CT angiography, the presence of HT on computer tomography (CT) scan after 24-48 hours from baseline, antithrombotic therapy before stroke, etiopathogenetic stroke subtype, secondary antithrombotic prophylaxis at discharge, in-hospital and 90-day mortality, and modified Rankin Scale (mRS) at discharge and at 90 days were analyzed. HT was defined symptomatic when associated to neurological worsening determining an increase of ≥ 4 points at NIHSS. Patients with HT were compared with those without HT. mRS at hospital discharge and at 90 days were the study outcomes. Poor prognosis was defined as mRS≥4.
Statistical analysis: Continuous variables were reported as mean ± standard deviation (SD) or as median and interquartile range (IQR) as appropriate. Categorical variables were analyzed using the χ2 test and Fisher’s exact test when appropriate. Univariate and multivariate logistic regression analyses were used to identify predictors of outcome; odds ratio (OR) and their 95th percentile confidence intervals (CI) were reported. A p value of <0.05 was considered statistically significant. All analyses were performed using MEDCALC statistical software (MedCalc Software Ltd, Acacialaan 22, B-8400 Ostend, Belgium).
Results
Five hundred and sixty-four patients (51.5% females) with mean age 77.5±11.8 years were the study population. Median pre-event mRS was 0 (IQR 0-0). Stroke subtypes were aterothrombotic: 10.8%, lacunar 23.9%, cardioembolic 30.6%, embolic of undetermined source (ESUS) 15.3%, undefined or other 19.1%. Fifty-two patients (9.2%) showed HT on brain CT performed 24-48 hours after baseline. In 28 patients (4.9%) HT was symptomatic. In patients with HT, history of previous transient ischemic attack (TIA)/stroke, known atrial fibrillation and heart failure were significantly more frequent than in patients without HT (Table 1).
No difference was found in NIHSS upon arrival between the two subgroups of patients, while at Stroke Unit admission median NIHSS was significantly higher in HT patients compared with those without [8(3-11) vs 3(1-5.25), p=0.0001]. Among patients with HT there was a higher percentage of cardioembolic strokes (59.6% vs 27.7%, p=0.0001) and of large vessel occlusion on CT angiography (44.2% vs 11.3%, p=0.0001) compared to patients without HT. The percentage of patients who underwent systemic thrombolysis, mechanical thrombectomy, or both was higher in patients with HT than in those without (Table 2).
Patients with HT had significantly higher length of hospitalization (LOS), in-hospital mortality, median mRS at discharge and at 90 days and higher percentage of mRS≥4 at discharge than patients without HT. HT patients were more frequently discharged without secondary antiplatelet or anticoagulant prophylaxis than those without (Table 3). The percentage of patients who died or with severe residual disability (mRS≥4) at discharge was significantly higher in patients with symptomatic HT than in those with asymptomatic HT (92.8% versus 45.8%, respectively, p=0.0028). In-hospital mortality was 17.8% in patients with symptomatic HT versus 12.5% in those with asymptomatic HT (p=0.7109).
The presence of HT was predictive of a negative outcome (mRS≥4 at discharge and at 90 days) with a good performance (AUC 0.720 and 0.776, respectively) (Figures 1a and 1b).
At multivariate analysis, the presence of HT was an independent predictor of negative outcome (mRS≥4), increasing the risk approximately four times both at discharge (OR 3.64, 95% CI 1.46-9.06) and at 90 days (OR 4.26, 95% CI 1.07-16.88). Dementia, creatinine clearance <50 ml/min, NIHSS≥8 at Stroke Unit admission, and increase of ≥4 points on NIHSS from emergency department arrival to Stroke Unit admission were additional predictive variables of negative outcome at discharge. Dementia and a NIHSS≥8 at Stroke Unit admission were also predictive variables of negative outcome at 90 days (Tables 4 and table 5).
Table 1: Cardiovascular risk factors and antithrombotic therapy of patients with and without hemorrhagic transformation.
| HT | no HT | p | |
|---|---|---|---|
| Number, % | 52(9.2) | 512(90.8) | |
| Sex, n(%) | 25(48) | 266(51.9) | 0.66 |
| Mean age ± SD, years | 75.9±15.5 | 77.7±11.4 | |
| Arterial hypertension, n(%) | 42(80.7) | 353(68.9) | 0.08 |
| Diabetes mellitus, n(%) | 12(23.0) | 143(29.8) | 0.51 |
| Previous TIA/stroke, n(%) | 19(36.5) | 111(21.6) | 0.023 |
| Carotid stenosis >50%/previous TEA, n(%) | 2(3.8) | 25(4.8) | 1 |
| Known atrial fibrillation, n(%) | 17(32.6) | 98(19.1) | 0.029 |
| Ischemic heart disease, n(%) | 6(11.5) | 62(12.1) | 1 |
| Heart failure, n(%) | 8(15.3) | 30(5.8) | 0.016 |
| Renal failure (CrCl<50 ml/min), n(%) | 10(19.2) | 93(18.1) | 0.47 |
| Dementia, n(%) | 7(13.4) | 53(10.3) | 0.75 |
| Previous antiplatelet therapy, n(%) | 17(32.6) | 158(30.8) | 0.26 |
| Previous anticoagulant therapy, n(%) | 9(17.3) | 60(11.7) | 0.35 |
| DOAC, n(%) | 5(9.6) | 30(5.8) | 0.54 |
| VKA, n(%) | 4(7.7) | 30(5.8) |
HT: Hemorrhagic Transformation; SD: Standard Deviation; TIA: Transient Ischemic Attack; TEA: Thromboendoarteriectomy; Crcl: Creatinine Clearance; DOAC: Direct Oral Anticoagulant; VKA; Vitamin K Antagonist.
Table 2: Clinical and neuroradiological characteristics in pa- tients with and without hemorrhagic transformation.
| HT | no HT | p | |
|---|---|---|---|
| NIHSS at ED arrival (median, IQR) | 3(0-5) | 3(2-7) | ns |
| NIHSS at SU admission (median, IQR) | 8(3-11) | 3(1-5.25) | 0.0001 |
| GCS at ED arrival (median, IQR) | 15(15-15) | 15(15-15) | ns |
| Cardioembolic etiology, n(%) | 31(59.6) | 142(27.7) | 0.0001 |
| Large vessel occlusion, n(%) | 23(44.2) | 58(11.3) | 0.0001 |
| Systemic thrombolysis and/or | |||
| Mechanical thrombectomy, n(%) | 17(32.6) | 72(14.0) | 0.0012 |
| Bilateral ischemic lesions, n(%) | 2(0.3) | 20(0.3) | 1 |
| Posterior circulation ischemic lesion, n(%) | 7(1.3) | 119(2.3) | 0.11 |
HT: Hemorrhagic Transformation; NIHSS: National Institutes Of Health Stroke Scale; ER: Emergency Department; IQR: Interquartile Range; SU: Stroke Unit; GCS: Glasgow Coma Scale; BP: Blood Pressure; SD: Standard Deviation.
Table 3: Outcome at discharge and at 90 days in patients with and without hemorrhagic transformation.
| HT | no HT | p | |
|---|---|---|---|
|
No secondary antithrombotic prophylaxis at discharge, n(%) |
17/44(38.6) | 59/490(12) | 0.0001 |
| In-hospital mortality, n(%) | 8(15.3) | 22(4.2) | 0.0036 |
| Length of stay, days (median, IQR) | 10.2±5.9 | 7.5±4.2 | 0.0001 |
| mRS alla dimissione (median, IQR) | 4(3-5) | 3(1-4) | 0.0001 |
| mRS ≥ 4 at discharge, n(%) | 38(73) | 192(37.5) | 0.0001 |
| 90-day mRS (median, IQR) | 4(3-5) | 2(1-3) | 0.0001 |
HT: Hemorrhagic Transformation; IQR: Interquartile Range; mRS: odified Rankin scale.
Table 4: Variables predictive of negative outcome (mRS≥4) at discharge.
| Variable | OR | 95% CI |
|---|---|---|
| Systemic thrombolysis and/or | 4.2615 | 1.0757-16.8820 |
| mechanical thrombectomy | 0.4167 | 0.2081 - 0.8345 |
| HT at 24/48 hours | 3.6439 | 1.4648-9.0643 |
| Posterior circulation stroke | 1.5195 | 0.9023-2.5589 |
| Bilateral stroke | 0.8582 | 0.3078-2.3928 |
| Female sex | 1.1754 | 0.7560-1.8277 |
| Large vessel occlusion | 1.5422 | 0.8389-2.8353 |
| NIHSS ≥ 8 at ED arrival | 0.5900 | 0.3320-1.0482 |
| NIHSS ≥ 8 at SU admission | 4.5742 | 2.0855-10.0329 |
| CrCl ≤ 50 ml/min | 2.3656 | 1.3681-4.0903 |
| Increase of NIHSS ≥ 4 between ED arrival | 0.8825 | 0.5567-1.3988 |
| and SU admission | 2.4046 | 1.0998-5.2575 |
| Age ≥ 75 years | 0.8825 | 0.5567-1.3988 |
| Dementia | 3.3167 | 1.5725-6.9953 |
| Diabetes mellitus | 1.1175 | 0.6803-1.8358 |
| Arterial hypertension | 1.2532 | 0.7762-2.0232 |
| Ischemic heart disease | 1.1043 | 0.5559-2.1937 |
| Known or newly diagnosed atrial fibrillation | 1.2300 | 0.7798-1.9400 |
| Platelets ≤ 150.000 | 0.8338 | 0.4283-1.6230 |
| PreviousTIA/stroke | 1.3161 | 0.7794-2.2226 |
| Cortical and/or cortical/subcortical stroke | 1.1319 | 0.7174-1.7861 |
mRS: modified Rankin scale; OR: Odds Ratio; CI: Confidence Interval; HT: Hemorrhagic Transformation; NIHSS: National Institutes of Health Stroke Scale; ED: Emergency Department; SU: Stroke Unit; CrCl: Creatinine Clearance; TIA: Transient Ischemic Attack.
Table 5: Variables predictive of negative outcome (mRS≥4) at 90 days.
| Variable | OR | 95% CI |
|---|---|---|
| HT at 24/48 hours | 4.2615 | 1.0757-16.8820 |
| Systemic thrombolysis and/or | 3.6439 | 1.4648-9.0643 |
| mechanical thrombectomy | 0.9209 | 0.2961-2.8637 |
| Posterior circulation stroke | 0.4755 | 0.1818-1.2433 |
| Bilateral stroke | 2.1350 | 0.4669-9.7624 |
| Female sex | 1.3163 | 0.6078-2.8510 |
| Large vessel occlusion | 0.4593 | 0.1277-1.6522 |
| NIHSS ≥ 8 at ED | 1.2198 | 0.4481-3.3203 |
| NIHSS ≥ 8 at SU admission | 24.7589 | 6.4885-94.4750 |
| CrCl ≤ 50 ml/min | 0.6263 | 0.1894-2.0718 |
| Increase of NIHSS ≥ 4 between ED arrival and | 0.8825 | 0.5567-1.3988 |
| SU admission | 0.7661 | 0.2018-2.9080 |
| Age ≥ 75 years | 0.5251 | 0.2302-1.1981 |
| Dementia | 22.8875 | 4.7970-109.1999 |
| Diabetes mellitus | 1.2389 | 0.5025-3.0543 |
| Arterial hypertension | 0.9873 | 0.4166-2.3398 |
| Ischemic heart disease | 1.5027 | 0.4496-5.0227 |
| Known or newly diagnosed atrial fibrillation | 1.9461 | 0.8864-4.2725 |
| Platelets ≤ 150.000 | 3.0167 | 0.9913-9.1806 |
| Pregvious TIA/stroke | 1.6293 | 0.6532-4.0641 |
| Cortical and/or cortical/subcortical stroke | 0.4604 | 0.1930-1.0985 |
mRS: modified Rankin scale; OR: Odds Ratio; CI: Confidence Interval; HT: Hemorrhagic Transformation; NIHSS: National Institutes of Health Stroke Scale; ED: Emergency Department; SU: Stroke Unit; CrCl: Creatinine Clearance; TIA: Transient Ischemic Attack.
Discussion
Despite HT is a well recognized entity in stroke medicine, it remains a poorly defined issue with many aspects of uncertainty. HT may influence the clinical course and prognosis of stroke. The interest on HT has grown in recent years, due to the widespread of systemic thrombolysis and/or mechanical thrombectomy. Our study showed that HT influences outcome and management of patients with acute ischemic stroke, representing an independent risk factor for poor prognosis, both in the short and long term. In our study HT was associated with a significantly longer LOS and a significantly lower percentage of patients discharged on secondary antithrombotic prophylaxis. Furthermore, our study highlighted a significant difference in the composite outcome in-hospital mortality/severe residual disability at discharge between patients with symptomatic and non-symptomatic HT. Although higher in percentage, the inhospital mortality of patients with symptomatic HT was not significantly higher compared to non-symptomatic HT.
The association between HT and negative prognosis was already known in the literature. Paciaroni M et al. showed a fifteen-time higher risk of mortality or severe disability in patients with parenchymal HT compared with patients without HT [2]. More recently, similar results were reported by D’Amelio M et al. showing an association between parenchymal HT and inhospital and three-month mortality [5], and Andrade JBC et al. who found that the presence of HT is associated with mortality, prolonged LOS, severe disability at discharge, risk of pneumonia and seizures, and that asymptomatic HT is associated with a more than five-fold risk of mRS≥4 at discharge [6]. Similar results were reported by Van Kranendonk KR et al. who found an association between HT and negative outcome, showing no significant differences in prognosis between patients with confluent petechial HT and those with parenchymal HT [7]. Gill D et al. showed that the development of post-thrombolysis parenchymal HT is associated with the absence of improvement or even neurological worsening (average increase of 7 points on NIHSS), in contrast to patients without HT, who show an improvement of post-thrombolysis NIHSS score in the large majority of cases [8]. In a study by Ge WQ et al. the NIHSS score 2 hours after thrombolysis and the presence of atrial fibrillation were risk factors for HT [9]. In our study NIHSS score ≥ 8 at Stroke Unit admission was an independent risk factor for poor in-hospital and 90-day outcome. Moreover, our study showed that an increase of more than 4 points of NIHSS from emergency department to Stroke Unit admission was also an independent risk factors for negative in-hospital outcome.
The management of antithrombotic secondary prophylaxis in patients with HT is controversial. While parenchymal HT, especially type II, should be considered as an intraparenchymal hemorrhage and managed accordingly, the management of petechial HT is more uncertain. Indeed, there are very few literature studies focusing attention on this topic. In the randomized controlled trial Tinzaparin in Acute Ischemic Stroke, asymptomatic petechial HT was found in approximately 30-35% of patients, with no significant differences between the aspirin arm and the medium and high dose tinzaparin arms, and no differences in terms of outcome between the three groups [10]. In another study by Kim JT et al. the introduction of secondary antithrombotic prophylaxis did not negatively affect the prognosis of patients with petechial HT [11]. However, the fear of further neurological deterioration represents the main reason for the delayed introduction of secondary antithrombotic prophylaxis in patients with HT. In the RAF and RAF-DOAC studies on more than 2000 patients with atrial fibrillation-related cardioembolic stroke, the incidence of HT was 11% (7.9% petechial, 3.1% parenchymal), and the presence of HT was associated with significantly higher mortality and disability [12]. The timing of initiation of secondary anticoagulant prophylaxis was on average twice longer in patients with HT than in those without HT (23.3 vs 11.6 days, respectively); however, the incidence of embolic recurrences at 90 days was comparable between the two groups (4.6% vs 4.9%, respectively) [12]. Our study showed similar results for patients with atrial fibrillation-related ischemic stroke. The most recent AHA/ASA guidelines, based on very limited available evidence, suggest the possibility of starting or continuing antithrombotic secondary prophylaxis in HT patients with a grade of recommendation IIb, weighing the clinical presentation and indications [13].
We recognized that our study has limitations mainly due to the retrospective and single center design. Despite these limitations, we think that it could add information about the topic.
Conclusion
HT represents the most feared complication of hyperacute phase of ischemic stroke being associated with higher mortality and residual disability. Our real life study confirms that HT is associated with a poor outcome in terms of mortality and severe residual disability, both in the acute phase and at 90 days and that HT represents an independent risk factor of poor outcome. Strategies aimed to reduce the incidence of HT and make an early diagnosis are warranted.
References
- Lindley RI, Wardlaw JM, Sandercock PA, et al. Frequency and risk factor for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis. 2004; 6: 235-246.
- Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate; predictive factor and influence on clinical outcome. Results of a prospective multicenter study. Stroke. 2008; 39: 2249-2256.
- Libman R, Kwiakowski T, Lyden P, et al. NINDS rt-PA Stroke Study Group. Asymptomatic hemorrhagic transformation of cerebral infarction does not worsen long-term outcome. J Stroke Cerebrovasc Dis. 2005; 2: 50-54.
- Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct. Relationship with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) Cohort. Stroke. 1999; 30: 2280-2284.
- D’Amelio M, Terruso V, Famoso G et al. Early and late mortality of spontaneous hemorrhagic transformation of ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23: 649-654.
- Andrade JBC, Mohr JP, Lima FO, et al. The Role of Hemorrhagic Transformation in Acute Ischemic Stroke Upon Clinical Complications and Outcomes. J Stroke Cerebrovasc Dis. 2020; 29: 104898.
- Van Kranendonk KR, Treurniet KM, Boers AMM, et al. MR CLEAN investigators. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg. 2019; 11: 464-468.
- Gill D, Baheerathan A, Aravind A, et al. Severe Hemorrhagic Transformation after Thrombolysis for Acute Ischemic Stroke Prevents Early Neurological Improvement. J Stroke Cerebrovasc Dis. 2016; 25: 2232-2236.
- Ge WQ, Chen J, Pan H, et al. Analysis of Risk Factors Increased Hemorrhagic Transformation after Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2018; 27: 3587-3590.
- England TJ, Bath PM, Sare GM, et al. TAIST Investigators. Asymptomatic hemorrhagic transformation of infarction and its relationship with functional outcome and stroke subtype: assessment from the Tinzaparin in Acute Ischemic Stroke Trial. Stroke. 2010; 41: 2834-2839.
- Kim JT, Heo SH, Park MS, et al. Use of antithrombotics after hemorrhagic transformation in acute ischemic stroke. PLoS One. 2014; 9: 89798.
- Paciaroni M, Bandini F, Agnelli G et al. Hemorrhagic Transformation in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Time to Initiation of Oral Anticoagulant Therapy and Outcomes. J Am Heart Assoc. 2018; 7: 010133.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019; 50: 344-418