SciBase Journals
SciBase Neurology
ISSN 2996-3788
- Article Type: Research Article
- Volume 1, Issue 2
- Received: Dec 12, 2023
- Accepted: Dec 27, 2023
- Published Online: Dec 29, 2023
Effects of Auditory Cue Length and Visual Cue Superimposition on Gait Initiation in Parkinson’s Disease: A Pilot Study
Takao Hashimoto*; Hiromasa Sato
Department of Neurology, Aizawa Hospital, Matsumoto, Japan.
*Corresponding Author: Takao Hashimoto
Department of Neurology, Aizawa Hospital, Matsumoto, Japan.
Tel: +81-263-33-8600;
Email: sinke-dr@ai-hosp.or.jp
Abstract
Objectives: Effects of cues on gait disturbance in Parkinson’s disease (PD) depend on features of cue presentation. We investigated the effects of the length of simple auditory cues on cue-triggered gait initiation in PD.
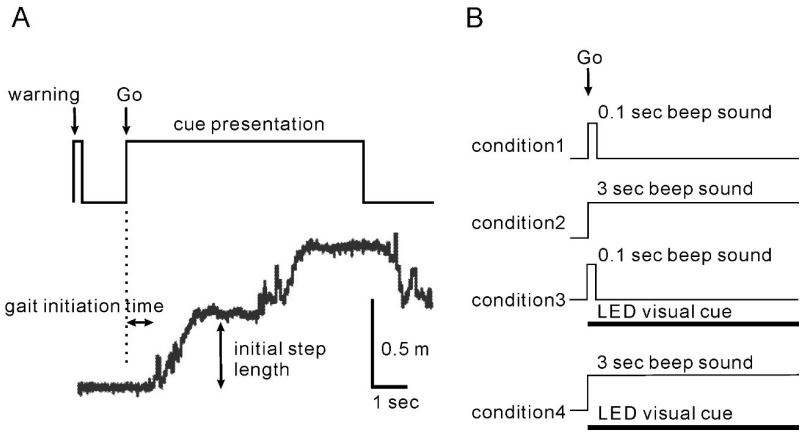
Methods: The subjects were 8 PD patients in OFF-state (6 women and two men, aged 50-75 years) and 8 normal controls (NCs) (5 women and three men, aged 55-77 years). For the assessment of gait initiation, the subjects were instructed to take three steps without the strong constraint of a quick start. The auditory cues consisted of long and short beeps, and the visual cue was a red light-emitting diode (LED) line projected across the floor 15 cm in front of the subjects’ toes. The four experimental conditions were: (1) short Beep condition (0.1 sec), (2) long Beep condition (3 sec), (3) short Beep/LED condition and (4) long Beep/LED condition.
Results: The gait initiation time was delayed, and the initial step length was short under the Beep conditions in the PD patients compared with those in the NCs. Neither parameter differed between the short Beep and the long Beep conditions. Superimposition of the visual cues improved the gait initiation time under the short Beep condition and the initial step length under the short and long Beep conditions.
Conclusion: The duration of single auditory cues does not contribute to the gait initiation time and initial step length in PD. Although large-sized studies are necessary to confirm the results, instantaneous auditory perception may be the primary driving force for the effects of single auditory cues on gait initiation.
Keywords: Cue; Gait; Freezing of gait; Parkinson’s disease.
Citation: Hashimoto T, Sato H. Effects of Auditory Cue Length and Visual Cue Superimposition on Gait Initiation in Parkinson’s Disease: A Pilot Study. SciBase Neurol. 2023; 1(2): 1010.
Introduction
Gait disturbance is one of the cardinal symptoms of Parkinson’s disease (PD), which significantly degrades the quality of life [1]. Parkinsonian gait involves several features: a reduction of gait speed, a reduction of step amplitude, gait festination, freezing of gait (FOG), balance deficit, postural abnormality, and so on [2,3]. Among the features of gait in PD, FOG is very troublesome, and it quickly leads to falls [4]. Cues of various modalities can resolve FOG or other features of gait disturbance and are adopted for rehabilitation of walking in PD [5,6]. Several studies or meta-analyses have been conducted to determine the superiority of cueing effects in treating gait disturbances in PD among cues of different modalities [7-11]. However, various features of cue presentation can affect the efficacy of the cue, and adjustment of the stimulus strength among cues of different types appears to be complicated. Piéron’s Law describes that simple reaction time decreases as a power function of stimulus intensity [12], and it was disclosed that not only perception but also force output in simple reaction tasks depends on stimulus intensity [13]. In tasks of gait initiation, many features of cue presentation other than simple stimulus intensity, for example, length of stimulus exposure, simultaneous multiple cues of different modalities, the three-dimensional position of stimulus to the subject, and so on, can affect cueing efficacy. As auditory cueing is used much more frequently than visual cueing in PD studies [10], we conducted a pilot study to investigate differences in the cueing effect on gait initiation between short and long auditory cues in PD patients.
Methods
The effects of length of auditory cue presentation and the effects of superimposition of 2 cues, auditory and visual cues, were studied, and the effects of cueing were compared between the normal control group and the Parkinson’s disease. The subjects were 8 PD patients in OFF-state (6 women and 2 men, aged 50-75 years) and 8 normal controls (NCs) (5 women and 3 men, aged 55-77 years). For the assessment of gait initiation, the subjects were instructed to take 3 steps without the strong constraint of a quick start. The auditory cues consisted of short and long beeps, and the visual cue was a red light-emitting diode (LED) line projected across the floor 15 cm in front of the subjects’ toes. The 4 experimental conditions were: (1) short Beep condition (0.1 sec), (2) long Beep condition (3 sec), (3) short Beep/LED condition and (4) long Beep/LED condition. Demographic data of the participants are presented in Table 1. Individuals with PD were recruited from the Department of Neurology, Aizawa Hospital, and age-matched controls were volunteers or spouses of participants with PD. PD patients were excluded if their scores on the Mini-Mental Status Examination were <24. The patients were tested in OFF-state in the daytime with their regular medications. All patients with PD were rated by investigators using the Unified Parkinson’s Disease Rating Scale [14] before the gait initiation test. The ethics committee of Aizawa Hospital approved the study for research on human subjects according to the Helsinki Declaration. All patients were fully informed of the procedure and consented to all aspects of this study.
The foot position was traced using a gait analysis device (Ultrasonic System DP-502080; ME, Inc., Matsumoto, Japan). The ultrasound transmitter was placed on the floor 2 m in front of the subject; the receiver was put on the subject’s shin at 20 cm above the floor, and the subject walked toward the transmitter. The distance between the transmitter and the receiver was converted to voltage at 30 times/sec, and the output voltage of the distance and the cue signals were fed into a computer with a sampling rate of 100 Hz. The distance was accurate to within a range of 0.5 cm. Electromyograms and center of foot pressure were not monitored.
The experimental setup and conditions are illustrated in Figure 1A. The subjects stood on a start line and gazed down at the floor before their feet. The subjects were instructed to take three steps with the left foot first after an auditory cue, following a preparatory vocal command, “ready,” given semi-randomly 2-3 sec before the cue signal. The subjects were instructed to “begin walking briskly in response to a cue sound” without the strong constraint of a quick start at the initial step. Auditory cues were a short beeping sound (a short Beep, 0.1-sec duration) and a long beeping sound (a long Beep, 3-sec duration). A visual cue was a red LED line, approximately 40 cm long, which was projected for 3 sec across the floor 15 cm in front of the subjects’ toes from the subjects’ right side. The beeping tone was 65 dB and 2300 Hz and was easily heard, and the LED line was seen by the subjects in the dim room. The auditory and visual cues were presented simultaneously. When an LED line was presented, the subjects were instructed to step over the LED line if the LED line appeared. The four conditions were randomly ordered, and two trials were carried out for each. The interval between the preparatory commands was 1-1.5 min, and the total time for the test was approximately 10 min. Two trials for each condition were collected, as trials >2 times in each condition have been associated with a risk of falls and fatigue for Hoehn & Yahr Stage IV patients. Sufficient rest was given between trials to avoid subject fatigue. The ability to hear the short beep sound, recognize the LED beam on the test floor, and ambulate three steps on the test floor without an assistive device was confirmed. All subjects were pre-trained with pretesting of >4 times until each fully understood the task. The measurement was started when the subject could perform the task without fail. The step parameters were as follows: the gait initiation time was defined as the period between the onset of the cues and the initial rising point of the foot position trace; the initial step length was defined as the distance between the initial start position and the plateau position.
Statistical tests were performed using the paired t-test for comparison of the results of the 4 cue conditions and the Mann-Whitney U test for comparison of the results of controls and PD. Bonferroni adjustment for multiple comparisons [15] was not applied, and the significant level was set at p-value ≤ 0.05 (one-tailed).
Results
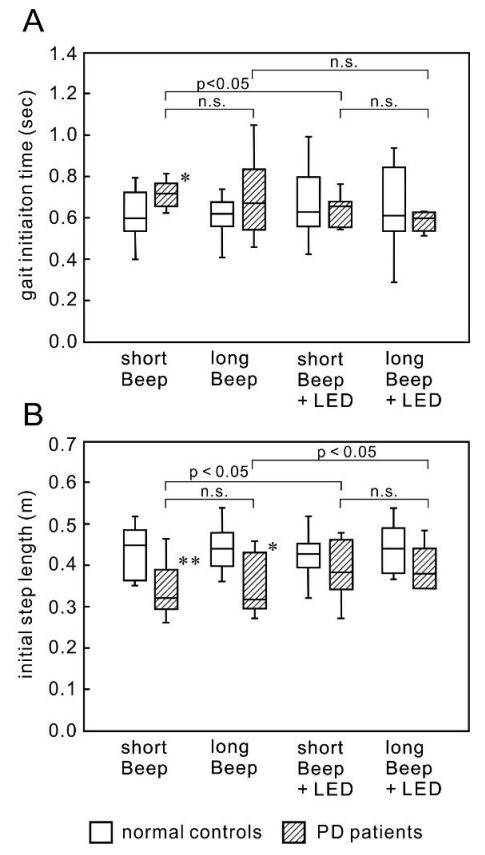
No PD patient showed freezing of gait, festination, shuffling, or fall during the task, although most patients showed freezing in daily walking. Figure 2 shows the results. The gait initiation time and the initial step length did not change in all conditions in the NCs. The gait initiation time under the short Beep condition was significantly longer in the PD patients (mean and SD, 0.71±0.07 sec, p=0.024) than in the NCs (0.61±0.13 sec). The gait initiation time under the long Beep condition was also longer in the PD patients (0.69±0.19 sec) than in the NCs (0.60±0.10 sec). Still, the difference was not significant because of a relatively large variation. The initial step length under the short Beep condition and that under the long Beep condition was significantly smaller in the PD patients (0.34±0.07 m, p=0.012 and 0.35±0.07 m, p=0.027, respectively) than in the NCs (0.43±0.06 m, 0.44±0.06 m, respectively).
Effects of auditory cue length: Both the gait initiation time and the initial step length were not different between the short Beep condition and the long Beep condition in PD (0.71±0.07 sec and 0.69±0.19 sec, respectively, for the gait initiation time and 0.34±0.07 m and 0.35±0.07, respectively for the initial step length) (Figure 2A).
Effects of visual cue superimposition: There was no difference in the gait initiation time between the short Beep condition with LED and without LED or between the long Beep condition with LED and without LED (0.61±0.13 sec and 0.62±0.12 sec, respectively, for the gait initiation time and 0.39±0.07 m and 0.39±0.05, respectively for the initial step length) (Figure 2A,B). The superimposition of LED increased the initial step length in the short Beep condition (from 0.34±0.07 m to 0.39±0.07 m, p = 0.08), but there was no significant difference. There was an increase in the initial step length in the long Beep condition by superimposition of LED with a significant difference (from 0.35±0.07 m to 0.39±0.05 m, p=0.030) (Figure 2B).
Table 1: Demographic data of participants.
| n, gender | age (yrs) |
height (cm) | weight (kg) | Hoehn & Yahr Stage (0-5) |
UPDRS III (0-108) | FOG UPDRS item (0-4) | Gait disturbances UPDRS item (0-4) |
|
|---|---|---|---|---|---|---|---|---|
| PD patients | 6F/2M | 65±7 | 159±12 | 57±17 | 29±06 (24) | 17±11 (5-33) | 18±13 (0-3) | 1.8±0.7 (1-3 ) |
| (50-72) | (140-175) | (41-90) | ||||||
| Normal controls | 5F/3M | 66±8 | 160±9 | 55±6 | ||||
| (55-77) | (141-172) | (45-63) |
Data are presented by means ± SD (ranges)
Box and whisker plots in all conditions are presented. (A) There was a decrease in the gait initiation time in both the short Beep condition and the long Beep condition with the superimposition of an LED cue, but it was insignificant. The gait initiation time was not different between the short and long Beep conditions. (B) There was a tendency toward an increase in the initial step length in both the short and long Beep conditions with the superimposition of an LED line. The initial step length was not different between the short Beep/LED condition and the long Beep/LED condition.
Discussion
It was confirmed that PD patients have a longer gait initiation time and a shorter initial step length on the short and long Beep conditions compared with NCs and that the superimposition of LED mostly normalizes these parameters. As there was no difference among the 4 conditions in NCs, these changes can be attributed to the deficits of the brain function in PD. Previous studies reported prolonged initiation time with a significant difference in PD [16-20]. We measured the gait initiation time of subjects without the strong constraint of a quick start. At the same time, most previous studies evaluated the shortest reaction time in gait under the instruction, “as quickly as possible”, and both reaction time paradigms demonstrated prolongation of the gait initiation time in PD. The initial step length under the Beep conditions was significantly smaller in the PD patients than in the NCs in the present study. Similarly, the decrease in initial step length in self-generated gait and externally triggered gait in PD has been commonly reported in previous studies [17,18]. Gait initiation typically consists of anticipatory postural adjustments (APAs) and the subsequent shifting of the center of pressure by stepping [21,22], and patients with PD tend to show impaired APAs [23].
The pivotal result is that the difference in the duration of the beep cues, 0.1 sec and 3 sec, produced no significant difference in the gait initiation time or the initial step length under the conditions with or without the superimposition of visual cues. These results suggest that all required to trigger gait may be instantaneous auditory perception and that the duration of the sound may not contribute to gait initiation. It is suggested that a separate mechanism is involved in initiating a motor command and in force production for the first step in APAs [26], and auditory cues may serve mainly to initiate a motor command. Once a motor command starts, the subsequent stepping procedure may automatically run without being affected by the extended cue presentation.
Concerning the effects of the superimposition of the visual cue on the cue of other modalities, the superimposition of the visual cue on the auditory cue improved the initial step length. It has been well recognized that visual and auditory cues improve the initial step length and strides in PD [5,6,9,24]; however, the effects of their superimposition on other sensory cues have rarely been studied, and such studies have not assessed the initial step. It was suggested that in a walking paradigm, auditory cues provide an external rhythm, compensating for the basal ganglia’s defective internal rhythm [25]. and that visual cues help to fill in for the motor set deficiency by providing visual data on appropriate stride length [26]. These findings provide the background to the different effects of the auditory and visual cues on the initial step length.
Although the results of this study lack sufficient case numbers to establish a conclusion of high reliability, they suggest the following: for Parkinson’s disease patients to overcome gait disturbances using cues and lead a normal daily life, it is imperative to explore more effective cueing mechanisms. While this study compared cueing effects based on the duration of auditory cues, further investigations need to encompass variations in sound volume, harmony, melody, and other auditory attributes. Additionally, it is essential to compare cueing effects across other modalities and explore the diverse changes inherent in the stimulus characteristics of each modality.
Conclusions
The results from the present study reveal that the duration of auditory cues does not contribute to the cueing effect on gait initiation in PD. Although large-sized studies are necessary to confirm the results, instantaneous auditory perception may be the major driving force for the effects of single auditory cues on gait initiation.
Declarations
Conflict of interest: The authors report no conflicts of interest.
Funding: This study was supported by no funding.
Acknowledgments: We thank Masao Kudo for technical assistance with step measurement.
References
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with morbidity and gait. Mov Disord. 2007; 22: 2192-95.
- Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996; 119: 551-68.
- Grabli D, Karachi C, Welter ML, Lau B, Hirsch EC et al. Normal and pathological gait: what we learn from Parkinson’s disease. J Neurol Neurosurg Psychiat. 2012; 83: 979-985.
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004; 19: 871-84.
- Rubinstein TC, Giladi N, Hausdorff JM. The power of cueing to circumbent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Mov Disord. 2002; 17: 1148-60.
- Nieuwboer A. Cueing for freezing of gait in patients with Parkinson’s disease: a rehabilitation perspective. Mov Disord. 2008; 23(2): 475-481.
- Lim I, van Wegen E, de Goede C, Deutekom M, Nieuwboer A et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin Rehabil. 205; 19: 695-713.
- Lohnes CA, Earhart GM. The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait Post. 2011; 33: 478-83.
- Spaulding SJ, Barber B, Colby M, Cormack B, Mick T, et al. Cueing and gait improvement among people with Parkinson’s disease: a meta-analysis. Arch Phys Med Rehabil. 2013; 94: 562-70.
- Rocha PA, Porfírio GM, Ferraz HB, Trevisani VFM. Effects of external cues on gait parameters of Parkinson’s disease patients: a systematic review. Clin Neurol Neurosurg. 2014; 124: 127-134.
- Lu C, Amundsen Huffmaster SL, Tuite PJ, Vachon JM, MacKinnon CD. Effect of cue timing and modality on gait initiation in Parkinson disease with freezing of gait. Arch Phys Med Rehabil. 2017; 98: 1291-99.
- Piéron H. Recherches sur les lois de variation des temps de latence sensorielle en function des intensités excitatrices. Année Psychol. 1914; 22: 17-96.
- Jaśkowski P, Rybarczyk K, Jaroszyk F, Lemański D. The effect of stimulus intensity on force output in simple reaction time tast\k in humans. Acta Neurobiol Exp. 1995; 55: 57-64.
- Fahn S, Elton RI, and members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health Care Information. 1987; 153-63.
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995; 310: 170.
- Elble RJ, Cousins R, Leffler K, Hughes L. Gait initiation by patients with lower-half parkinsonism. Brain. 1996; 119: 1705-16.
- Rosin R, Topka H, Dichgans J. Gait initiation in Parkinson’s disease. Mov Disord 1997; 12: 682-90.
- Halliday SE, Winter DA, Frank JS. Gait initiation in Parkinson’s disease subjects. Gait Post. 1998; 8: 8-14.
- Dibble LE, Nicholson DE, Schultz B, MacWilliams BA, Marcus RL, et al. Sensory cueing effects on maximal speed gait initiation in persons with Parkinson’s disease and healthy elders. Gait Post. 2004; 19: 215-25.
- Smulders K, Esselink RA, Bloem BR, Cool R. Freezing of gait in Parkinson’s disease is related to impair motor switching during stepping. Mov Disord. 2015; 30: 1090-97.
- Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. J Physiol. 1991; 437: 635-53.
- Elble RJ, Moody C, Leffler K, Sinha R. The initiation of normal walking. Mov Disord. 1994; 9: 139-46.
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997; 12: 206-15.
- Ying Jiang, Kathleen E Norman. Effects of visual and auditory cues on gait initiation in people with Parkinson’s disease. Clin Rehabil. 2006; 20: 36-45.
- McIntosh GC, Brown SH, Rice RR, Thaut MH. Rhythmic auditorymotor facilitation of gait patterns in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997; 62: 22-26.
- Morris ME, Iansek R, Galna B. Gait festination and freezing in Parkinson’s disease: pathogenesis and rehabilitation Mov Disord. 2008; 23 (2): 451-460.