SciBase Journals
SciBase Neurology
ISSN 2996-3788
- Article Type: Research Article
- Volume 2, Issue 1
- Received: Jan 03, 2024
- Accepted: Feb 15, 2024
- Published Online: Feb 22, 2024
Seizures in Multiple Sclerosis are, above all, a Matter of Brain Viability
Anamarija Kavčič1*; Werner E Hofmann2
1District Hospital Lohr, Psychiatric Clinic Aschaffenburg, Am Hasenkopf 3, 63739 Aschaffenburg, Germany
2Outpatient Clinic for Neurology, Psychiatry and Psychotherapy, Weichgasse 5, 63868 Großwallstadt, Germany.
*Corresponding Author: Anamarija Kavčič
District Hospital Lohr, Psychiatric Clinic Aschaffenburg, Am Hasenkopf 3, 63739 Aschaffenburg, Germany.
Email: anamarija.kavcic@gmx.de
Abstract
Objectives: Most patients with Multiple Sclerosis (MS) never have a seizure, although they are at risk for seizures since the onset of MS. This paradox leads us to suppose that MS plays a minor role in seizure generation and epileptogenesis.
Methods: Qualitative study using data triangulation and inductive content analysis. A comprehensive literature search was carried out in four electronic databases (MEDLINE, Embase, Web of Science, and Google Scholar).
Results: In MS patients, unprovoked seizures occur rarely (in about 3 percent of cases). Pediatric MS involves seizures more common than adult or late-onset MS. In general, children with seizures do not have poorer MS prognoses than children without seizures. Contrary to the general population, seizures do not peak in older MS patients. Since the use of disease-modifying therapies in the treatment of MS (i.e., since 1993), the frequency of seizures in MS patients has not changed considerably. Epilepsy syndrome cannot be recognized in MS patients with seizures. As a rule, seizures in MS are not difficult to treat. A sudden unexpected death in MS patients with seizures has not been observed more commonly than in the general epilepsy population. A disruption of the blood–brain barrier is the most obvious proconvulsive factor of MS. However, neither MS relapses nor a high rate of MS relapses is normally accompanied by seizures. Structural brain abnormalities usually accumulate over the course of MS. However, a high brain magnetic resonance imaging lesion load does not have a substantial impact on seizure occurrence in MS.
Conclusion: MS does not have a major role in seizure generation and epileptogenesis. In most cases, the seizure-promoting effects of MS can be successfully counteracted by the brain´s protective mechanisms.
Keywords: Seizures; Epilepsy; Multiple sclerosis; Brain viability; Blood-brain-barrier; Neurohomeostasis; Autoimmunity; Structural brain abnormalities.
Abbreviations: BBB: Blood-Brain-Barrier; CNS: Central Nervous System; MRI: Magnetic Resonance Imaging; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; SPMS: Secondary Progressive MS.
Citation: Kavčič A, Hofmann WE. Seizures in Multiple Sclerosis are, above all, a Matter of Brain Viability. SciBase Neurol. 2024; 2(1): 1011.
Introduction
The epileptogenicity of Multiple Sclerosis (MS) was realized shortly after Charcot defined MS and gave it the name [1,2]. At that time (i.e., in the second half of the 19th century), the occurrence of seizures in MS patients was observational evidence that, due to a lack of understanding of both MS and seizure disorders, could not evoke a debate on the causation of seizures in MS. Since then, a mounting number of epidemiological studies have shown an increased frequency of epilepsy in MS patients compared with the general population [3,7]. Some researchers have tried to prove the opposite by intensive searching for competing causes of seizures in MS patients [5]. However, even though they found competing risks of seizures in many cases,they could not demonstrate that seizures in MS happen purely by chance [5]. The seizure generation and epileptogenesis in MS are still poorly understood [7]. During an MS relapse, epileptic seizures do not usually occur [8]. A relapse rate in MS does not correlate with seizure occurrence [9]. Owing to these observations, it seems that highly active MS does not have a proconvulsive effect. Current knowledge on the Blood–Brain Barrier (BBB) argues the opposite [10,11]. Namely, every MS relapse implies a breakdown of the BBB [11], and this—as has been repeatedly demonstrated by miscellaneous experimental studies—actually induces seizures [10]. A lack of coherence between seizure occurrence and MS activity leads us to suppose that MS has a minor role in seizure generation and epileptogenesis.
Methods
To elucidate the role of MS in seizure generation and epileptogenesis, a qualitative study using data triangulation and inductive content analysis was performed. A comprehensive literature search was conducted in four electronic databases (MEDLINE, Embase, Web of Science, and Google Scholar). The following keywords or key phrases were searched – multiple sclerosis, clinically isolated syndrome, radiologically isolated syndrome, seizure generation, epileptogenesis, risk factors for seizures, structural brain abnormalities, autoimmunity, blood– brain barrier, blood–brain barrier disruption, excitatory–inhibitory balance, homeostasis, brain aging, neurodegeneration, immunosenescence. To avoid ambiguities regarding seizures and epilepsy in MS patients (see Limitations), in the study, we preferred the term seizures. We covered all epileptic seizure disorders that could be related to MS. Acute symptomatic seizures and genetic epilepsies in MS patients were not addressed. The term epilepsy was used only when it was inevitable due to context.
Results
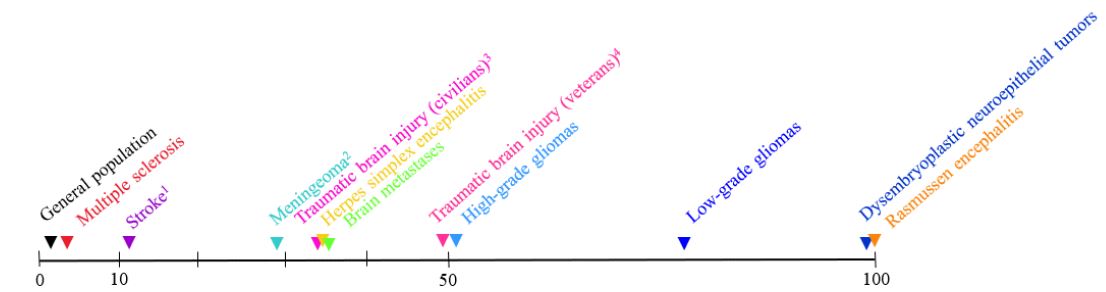
Recent epidemiological studies have shown that epilepsy occurs in approximately 3% of MS patients [6,7]. A comparison to the general population and to some other patients is depicted in Figure 1 [12,17].
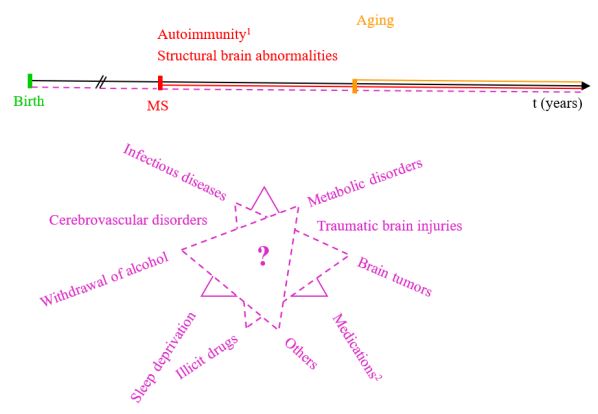
Possible causes of seizures in MS: It is certain that MS bears a risk factor for seizures because (1) seizure occurrence is considerably higher in MS patients than in the general population [7], and (2) structural brain abnormalities existing in every MS patient usually accumulate over the course of MS [18]. If MS is an autoimmune disease, autoimmunity must be considered a possible cause of seizures as well [19]. Furthermore, while living with the disease, MS patients are aging (an additional risk factor for seizures) [20]. MS patients can suffer from medical conditions unrelated to MS, which concurrently carry a risk of seizures (Figure 2) [20]. Considering the general knowledge on the genetics of epilepsy [21], some MS patients are very probably genetically predisposed to seizures. The current diagnostic methods for seizures are insufficient to bridge the gap between the risk factors for seizures (population level) and the actual cause of seizures (individual level) [22]. Therefore, the true cause of seizures in a particular MS patient is seldom, if ever, absolutely clear.
Proconvulsive properties of structural brain abnormalities: Gray and white matter abnormalities are present not only in structural epilepsies but also in other epilepsies, including idiopathic generalized epilepsies [23,24]. Their role in seizure generation and epileptogenesis remains incompletely understood [24].
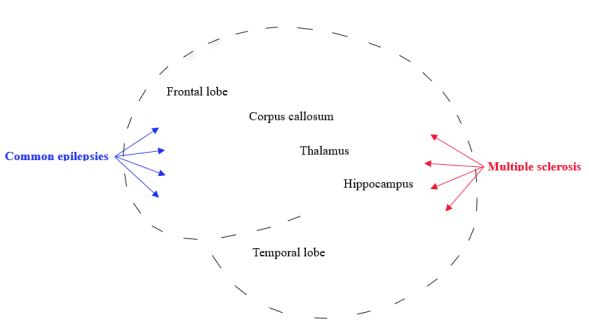
The frequent structural brain abnormalities in both the epilepsies and the MS are represented in Figure 3. Changes in the frontal lobe and thalamus are usually seen in temporal lobe epilepsy (the most common type of focal epilepsy syndrome), idiopathic generalized epilepsies, and MS [24,25]. Hippocampal sclerosis, one of the most common pathological features in drug-resistant epilepsy, is found in up to 45% of all epilepsy syndromes [26]. In MS, regional atrophy of the hippocampus can already be identifiable in the early clinical stages [27]. Hereafter, across the MS course, the structural alterations of the hippocampi (including demyelination, synaptic dysfunction, and neuronal loss) often progress [27]. Developmental anomalies of the corpus callosum are frequently associated with seizures [28]. Callosotomy can improve seizure outcomes in some drugresistant generalized epilepsies [29]. In MS, changes in the corpus callosum can be seen early in the disease course and vary according to the duration and severity of the disease, from focal demyelination to almost complete destruction of the corpus callosum [30].
Proconvulsive properties of autoimmunity: Since the 1960s, several antibody-mediated encephalitis accompanied by seizures have been demonstrated [19]. In MS patients with seizures, no specific autoantibody has yet been identified [19]. Consequently, the contribution of immunological alterations to seizure generation and epileptogenesis in MS cannot be directly evidenced. However, there have been anecdotic reports on seizure control in MS through immunotherapy after standard antiepileptic drugs have failed to suppress seizures [31,32]. Hence, at least in some MS cases, the immunological etiology of seizures cannot be denied.
Resilience to seizures in MS: The infrequency of seizures in MS patients proves that in MS, the regulation of neuronal excitability is mainly preserved [7,33]. Proconvulsive effects of MS are, in most cases—due to the brain´s protective mechanisms—successfully counteracted. This phenomenon can be seen throughout the course of MS. The onset of MS is clinically silent [34]. MS relapses are normally not accompanied by seizures [8]. There is a whole range of MS unrelated risk factors for seizures (Figure 2), which—alone or in combination—are frequently experienced by MS patients, but below them, MS patients rather infrequently develop a seizure [20]. In the later stages of MS, seizures do not peak [6].
First-time seizures, in which evaluation reveals MS, at first glance question the resilience of MS patients to seizures. However, it is seen only in about 8% of MS cases with unprovoked seizures that a first-time seizure represents the first clinical manifestation of MS [4]. These kinds of seizures in MS are very probably overestimated because of the lack of evidence-based guidelines for evaluation after a first seizure in an MS patient [5]. So far, if MS is diagnosed, a first-time seizure is usually explained by MS without any further evaluation, although every seemingly unprovoked seizure in an MS patient is not always caused by MS [35]. In addition, first-time seizures in patients newly diagnosed with MS are not more difficult to treat than other seizures in MS [4]. They by no means herald drug-resistant epilepsy.
Children are more prone to seizures than adults: This observation from the general population has also been observed in the pediatric MS population [12,36]. The occurrence of epilepsy in children with MS is considerably higher than in patients with adult-onset MS but is still rare; namely, less than 10% of children with MS suffer from epilepsy [36]. Epilepsy in a child with MS (which is very probably a result of MS) does not portend poor outcomes, neither with regard to epilepsy nor to MS. Seizures in children with MS are usually pharmacoresponsive [36]. An accumulation of disability in children with MS due to seizures has not been observed [36].
Current therapies for MS do not seem to affect seizure occurrence: Although disease-modifying therapies for MS (in clinical use since 1993) have reduced disability accrual in relapsing-remitting MS (RRMS) patients, the occurrence of seizures in RRMS patients has not changed noticeably in the last three decades [6,37].
Convulsive status epilepticus is not a frequent event in MS patients: In MS patients, convulsive status epilepticus occurs very rarely [32]. The treatment of status epilepticus in MS patients usually does not pose particular difficulties [32]. In refractory status epilepticus, the addition of immunotherapy often proves beneficial [32].
In MS patients without a prior history of seizures, status epilepticus carries an 86% risk of epilepsy over the subsequent 10 years [9]. The possible correlation between status epilepticus and subsequent pharmacoresistant epilepsy in MS patients has not yet been studied. However, pharmacoresistant epilepsy has not been more commonly observed in MS patients than in the general population [3].
Sudden unexpected deaths in MS patients: Sudden unexpected death in MS patients with seizures has not yet been widely investigated. There are no hints that in MS patients with seizures, sudden unexpected death would be more common than in the general epilepsy population [38].
The role of MS in seizure generation and epileptogenesis: The natural history of seizures in MS shows that it does not play a major role in seizure generation or epileptogenesis. This view is confirmed by epidemiological data on seizures and epilepsy in MS patients [7]. Specific pathological events underlying MS are seizure-promoting [11]. However, in vivo, they can be successfully counteracted by the brain´s protective mechanisms [33,39].
1within 5 years after stroke
2preoperative
3within 5 years after injury
410 or more years after injury
1if MS is an autoimmune disease
2with a low threshold for seizures
Discussion
The BBB disruption—the most striking seizure-promoting effect of MS—highlights the paradoxical feature of MS patients – resilience to seizures [11]. Most MS patients never have a seizure, even most of them with a high brain Magnetic Resonance Imaging (MRI) lesion load or a long disease duration [7,40]. The homeostatic regulation of neuronal excitability is usually maintained in MS, although brain integrity is—as a result of MS—irreversibly lost [25]. How this is possible, however, is still poorly understood.
The nervous system mitigates its devastation due to MS: The human nervous system possesses numerous protective and regenerative mechanisms [39,41]. They invariably shape the MS manifestation and course. Here, interindividual variability must be considered because the ability of the Central Nervous System (CNS) to maintain itself is genetically regulated and, additionally, influenced by the environment [39,42]. Every MS relapse with a complete neurological recovery and therapy naïve benign MS indirectly proves the beneficial contribution of the CNS in the manifestation of MS. However, in progressive MS, CNS viability prevails over the devastating consequences of MS as well. On account of accumulating neurological disability, the protective and regenerative processes in progressive MS are admittedly not easily recognizable. Yet they certainly exist. Otherwise, there would be no MS patients who would live for years in spite of MS progression. Nevertheless, a great number of patients with progressive MS reach old age [43].
Regulation of neuronal excitability is profoundly protected: The surprisingly rare occurrence of seizures in MS reveals that the regulation of neuronal excitability—the basic prerequisite of normal brain function in humans—does not automatically fail when the brain structure is irreversibly impaired due to MS. A similar uncoupling of neuronal excitability from brain intactness is seen—to a greater or lesser extent—in other acquired diseases of the brain, in stroke, and in traumatic brain injury (Figure 1) [13,15,17]. Owing to such a hierarchy in brain organization, brain viability and functionality are much greater than they could be if the regulation of neuronal excitability is coupled with the normal brain structure. However, in spite of this and other major strengths, the human brain has several (minor) weaknesses on which environmental threats or unhealthy habits play, and, finally, often cause various diseases of the brain.
MS is a risk factor for seizures: Although the exact origin of MS still needs to be clarified, it is evident that MS is a disease with two protagonists—the central nervous system and the immune system. Both are never the same, for they are—owing to their fundamental aim—extremely open to the outside world (which is mutable and frequently dangerous) and because they memorize [39,44]. Viewed in this light, the variability in the clinical manifestation of MS appears to be a logical consequence of the numerous interactions between the two changeable organic systems
Epidemiological data on a considerably higher frequency of seizures in MS patients compared to the general population [7] raise the question how MS causes seizures. However, this question disregards one of the core features of seizures in MS—their rarity. Namely, less than 10 percent of MS patients experience seizures [7], although in all MS patients, the brain is ad infinitum—and often mounting—affected owing to MS [25,27,30].
Generally speaking, the occurrence of seizures implies a breakdown in the regulation of neuronal excitability [33]. Why it happened only in some MS patients is still an enigma. Yet the current knowledge on seizures in MS portends that the brain, with its viability—and not the pathological processes related to MS per se—rules whether seizures occur in MS or not.
Occurrence of seizures in MS is a complex phenomenon: In MS patients with seizures, all seizure types (epilepsy types) can be seen [3]. Here, epilepsy syndrome cannot be recognized. Consequently, it must be assumed that several pathophysiological mechanisms underlie seizure generation and epileptogenesis in MS. This reasoning is strongly supported by the age of seizure onset, per se, and in relation to MS duration. In three and more decades, while living with MS, neither the CNS of MS patients nor their MS remained the same. The brain develops and subsequently ages (pediatric-onset MS) or ages (adult- and late-onset MS), and, in addition, usually suffers losses as a result of destructive processes related to MS (primary progressive MS and secondary progressive MS (SPMS)) [42,45]. With the passing of time, MS activity usually wanes, although the disability in MS patients often accumulates [45,46]. Therefore, it is impossible that a first-ever unprovoked seizure in a child with MS, in a young adult newly or recently diagnosed with MS, or in an old person with long-term MS could share the same underlying mechanism.
Given that MS relapses are practically never accompanied by seizures [8], MS patients with seizures cannot be viewed as those with less efficient endogenous antiseizure mechanisms. If an acute neurologic deterioration (with BBB disruption, as is the case in MS relapses [11]) proceeds without seizures, then the endogenous antiseizure mechanisms are certainly efficient.
An overcharging of the endogenous antiseizure mechanisms could explain the occurrence of every single seizure in MS patients. However, a lack of clear correlation between seizure occurrence and brain MRI lesion load or MS duration [7,47] argues against the assumption that MS per se might, due to its intensity or over the course of time, induce exhaustion of the endogenous antiseizure mechanisms.
A lesson on corpus callosum: The non-occurrence of seizures in MS, usually seen also in progressive MS [9], reminds by itself that the occurrence of seizures in MS cannot be generally explained by an exhaustion of the endogenous antiseizure mechanisms. How it is possible that the endogenous antiseizures mechanisms in MS do not abate as a rule still needs to be clarified. However, the changes in the corpus callosum in MS (varying from small scattered foci of demyelination to almost complete destruction [30]) provide food for thought. An extensive destruction of the corpus callosum taking place in advanced MS is actually like a corpus callosotomy (a surgical procedure often efficiently used in the treatment of patients with drug-resistant epilepsy since 1940 [29]).
Besides the destruction of the corpus callosum, there have to be other MS-related changes in the CNS that are unfavorable for seizure generation and propagation in MS. Keep in mind that all stages of MS are features of resilience to seizures [7,9] and not only the late stages.
Limitations
Owing to its analytical approach, this study has no considerable limitations. Nevertheless, it should be considered that the field of both MS and epilepsy has been affected by the evolution of their understanding and by diagnostical errors due to a lack of specific diagnostic tests for them. Several diseases mimic MS [48]. In some cases, they were mistakenly diagnosed with MS, especially before the MRI era (i.e., before the 1980s [49]). Similarly, the diagnosis of epileptic seizures and epilepsy in MS patients can be challenging. Nonepileptic paroxysmal events (resembling epileptic seizures) are sometimes misdiagnosed as epileptic seizures [22]. Furthermore, the definition of epilepsy has changed. According to the current definition of epilepsy [50], all MS patients cannot be diagnosed with epilepsy after the first unprovoked seizure but only SPMS patients and those in which a first-ever seizure leads to status epilepticus [9]. Due to this most recent and all prior revisions of the definition of epilepsy, the comparison of epidemiological studies on epilepsy in MS over time is doubtlessly restricted. In addition, apparently unprovoked seizures in MS are not always related to MS, although they are often automatically (without any extended diagnostic) viewed as a result of MS [35].
Conclusions
The seizure generation and epileptogenesis in MS are still poorly understood. MS possesses proconvulsive properties. The destruction of BBB is the most obvious seizure-promoting factor of MS. Yet a high MS activity (clinically manifested as MS relapses and invariably accompanied by BBB destruction) does not correlate with seizure occurrence. Like most patients with non-progressive MS, most patients with progressive MS never have a seizure. Viewed in this light, it is evident that MS per se cannot play a major role in seizure generation and epileptogenesis.
A deep consideration of the cause of seizures in MS patients guides toward the homeostatic regulation of neuronal excitability. If it fails, seizures are an unavoidable event. The frequency of seizures in MS of less than 10 percent indicates that in human beings, the homeostatic regulation of neuronal excitability is not coupled to structural intactness of the brain. The same can be seen in other acquired brain diseases and traumatic brain injuries as well.
Seizure occurrence in MS is dependent much more on the innate brain´s capabilities to inhibit seizure generation and epileptogenesis than on MS per se. Here, it clearly shows that the manifestation of MS is significantly shaped by neurohomeostasis. Therefore, without understanding neurohomeostasis (in health and disease), the heterogeneity of MS cannot be understood.
References
- Leube W. Ueber multiple inselförmige Sklerose des Gehirns und Rückenmarks. Deutsches Arch. f. klin. Med. VIII. Bd. 1871: 1-29.
- Zalc B. One hundred and fifty years ago Charcot reported multiple sclerosis as a new neurological disease. Brain. 2018; 141(12): 3482-3488.
- Koch M, Uyttenboogaart M, Polman S, De Keyser J. Seizures in multiple sclerosis. Epilepsia. 2008; 49(6): 948-953.
- Sponsler JL, Kendrick-Adey AC. Seizures as a manifestation of multiple sclerosis. Epileptic Disord. 2011; 13(4): 401-410.
- Neuß F, von Podewils F, Wang ZI, Süße M, Zettl UK, Grothe M. Epileptic seizures in multiple sclerosis: prevalence, competing causes and diagnostic accuracy. J Neurol. 2021; 268(5): 1721-1727.
- Mahamud Z, Burman J, Zelano J. Temporal trends of epilepsy in multiple sclerosis. Acta Neurol Scand. 2022; 146(5): 492-498.
- Kuntz S, Wu AS, Matheson E, Vyas I, Vyas MV. Association between multiple sclerosis and epilepsy: A systematic review and meta-analysis. Mult Scler Relat Disord. 2023: 104421. doi: 10.1016/j.msard.2022.104421. Epub 2022 Nov 19. PMID: 36434909.
- Kalincik T. Multiple Sclerosis Relapses: Epidemiology, Outcomes and Management. A Systematic Review. Neuroepidemiology. 2015; 44(4): 199-214.
- Mahamud Z, Burman J, Zelano J. Risk of epilepsy after a single seizure in multiple sclerosis. Eur J Neurol. 2018; 25(6): 854-860.
- Greene C, Hanley N, Reschke CR, Reddy A, Mäe MA, Connolly R, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022; 13(1): 2003. doi: 10.1038/s41467-022-29657-y. PMID: 35422069; PMCID: PMC9010415.
- Balasa R, Barcutean L, Mosora O, Manu D. Reviewing the Significance of Blood-Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int J Mol Sci. 2021; 22(16): 8370. doi: 10.3390/ijms22168370. PMID: 34445097; PMCID: PMC8395058.
- Beghi E. The Epidemiology of Epilepsy. Neuroepidemiology. 2020; 54(2): 185-191.
- Ertürk Çetin Ö, İşler C, Uzan M, Özkara Ç. Epilepsy-related brain tumors. Seizure. 2017; 44: 93-97.
- Fordington S, Manford M. A review of seizures and epilepsy following traumatic brain injury. J Neurol. 2020; 267(10): 3105-3111.
- Galovic M, Ferreira-Atuesta C, Abraira L, Döhler N, Sinka L, Brigo F, et al. Seizures and Epilepsy After Stroke: Epidemiology, Biomarkers and Management. Drugs Aging. 2021; 38(4): 285-299.
- Tang C, Yang W, Luan G. Progress in pathogenesis and therapy of Rasmussen’s encephalitis. Acta Neurol Scand. 2022; 146(6): 761-766.
- Hersh N, Ben Zvi H, Goldstein L, Steiner I, Benninger F. Epilepsy following herpes simplex encephalitis - A case series. Epilepsy Res. 2023; 192: 107137. doi: 10.1016/j.eplepsyres.2023.107137. Epub 2023 Apr 5. PMID: 37060749.
- Tedeschi G, Dinacci D, Comerci M, Lavorgna L, Savettieri G, Quattrone A, et al. Brain atrophy evolution and lesion load accrual in multiple sclerosis: a 2-year follow-up study. Mult Scler. 2009; 15(2): 204-211.
- Geis C, Planagumà J, Carreño M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. 2019; 129(3): 926-940.
- Reinecke LCS, Doerrfuss JI, Kowski AB, Holtkamp M. Acute symptomatic seizures in the emergency room: predictors and characteristics. J Neurol. 2022; 269(5): 2707-2714.
- Ruggiero SM, Xian J, Helbig I. The current landscape of epilepsy genetics: where are we, and where are we going? Curr Opin Neurol. 2023; 36(2): 86-94.
- Leibetseder A, Eisermann M, LaFrance WC Jr, Nobili L, von Oertzen TJ. How to distinguish seizures from non-epileptic manifestations. Epileptic Disord. 2020; 22(6): 716-738.
- Nuyts S, D’Souza W, Bowden SC, Vogrin SJ. Structural brain abnormalities in genetic generalized epilepsies: A systematic review and meta-analysis. Epilepsia. 2017; 58(12): 2025-2037.
- Whelan CD, Altmann A, Botía JA, Jahanshad N, Hibar DP, Absil J, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain. 2018; 141(2): 391-408.
- Popescu BF, Lucchinetti CF. Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol. 2012; 12: 11. doi: 10.1186/1471-2377-12-11. PMID: 22397318; PMCID: PMC3315403.
- Thom M. Review: Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol. 2014; 40(5): 520-543.
- Rocca MA, Barkhof F, De Luca J, Frisén J, Geurts JJG, Hulst HE, et al. The hippocampus in multiple sclerosis. Lancet Neurol. 2018; 17(10): 918-926.
- Unterberger I, Bauer R, Walser G, Bauer G. Corpus callosum and epilepsies. Seizure. 2016; 37: 55-60.
- Markosian C, Patel S, Kosach S, Goodman RR, Tomycz LD. Corpus Callosotomy in the Modern Era: Origins, Efficacy, Technical Variations, Complications, and Indications. World Neurosurg. 2022; 159: 146-155.
- Barnard RO, Triggs M. Corpus callosum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1974; 37(11): 1259-1264.
- Sotgiu S, Murrighile MR, Constantin G. Treatment of refractory epilepsy with natalizumab in a patient with multiple sclerosis. Case report. BMC Neurol. 2010; 10:84. doi: 10.1186/1471-2377-10-84. PMID: 20863362; PMCID: PMC2954970.
- Atmaca MM, Gurses C. Status Epilepticus and Multiple Sclerosis: A Case Presentation and Literature Review. Clin EEG Neurosci. 2018; 49(5): 328-334.
- Marom S. Emergence and maintenance of excitability: kinetics over structure. Curr Opin Neurobiol. 2016; 40: 66-71.
- Marrie RA, Allegretta M, Barcellos LF, Bebo B, Calabresi PA, Correale J, et al. From the prodromal stage of multiple sclerosis to disease prevention. Nat Rev Neurol. 2022; 18(9): 559-572.
- Zimatore DS, Trentadue M, Castellaro M, Ferlisi M, Piovan E, Calabrese M. A case of epilepsy in multiple sclerosis: Three-dimensional double inversion recovery sequences revealed cortical dysplasia. Neuroradiol J. 2017; 30(4): 352-355.
- Chabas D, Strober J, Waubant E. Pediatric multiple sclerosis. Curr Neurol Neurosci Rep. 2008; 8(5): 434-441.
- Claflin SB, Tan B, Taylor BV. The long-term effects of disease modifying therapies on disability in people living with multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord. 2019; 36: 101374. doi: 10.1016/j.msard.2019.08.016. Epub 2019 Aug 15. PMID: 31450158.
- Mahamud Z, Burman J, Zelano J. Prognostic impact of epilepsy in multiple sclerosis. Mult Scler Relat Disord. 2020; 38: 101497. doi: 10.1016/j.msard.2019.101497. Epub 2019 Nov 5. PMID: 31726355.
- Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron. 2016; 89(2): 248-268.
- Grothe M, Ellenberger D, von Podewils F, Stahmann A, Rommer PS, Zettl UK. Epilepsy as a predictor of disease progression in multiple sclerosis. Mult Scler. 2022; 28(6): 942-949.
- Traiffort E, Kassoussi A, Zahaf A, Laouarem Y. Astrocytes and Microglia as Major Players of Myelin Production in Normal and Pathological Conditions. Front Cell Neurosci. 2020; 14: 79. doi: 10.3389/fncel.2020.00079. PMID: 32317939; PMCID: PMC7155218.
- MacDonald JL, Tharin S, Hall SE. Epigenetic regulation of nervous system development and function. Neurochem Int. 2022; 152: 105249. doi: 10.1016/j.neuint.2021.105249. Epub 2021 Nov 24. PMID: 34826529.
- Walz L, Brooks JC, Shavelle RM, Robertson N, Harding KE. Life expectancy in multiple sclerosis by EDSS score. Mult Scler Relat Disord. 2022; 68: 104219. doi: 10.1016/j.msard.2022.104219. Epub 2022 Oct 5. PMID: 36244189.
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020; 20(6): 375-388.
- Lublin FD, Häring DA, Ganjgahi H, Ocampo A, Hatami F, Čuklina J, et al. How patients with multiple sclerosis acquire disability. Brain. 2022; 145(9): 3147-3161.
- McFaul D, Hakopian NN, Smith JB, Nielsen AS, Langer-Gould A. Defining Benign/Burnt-Out MS and Discontinuing DiseaseModifying Therapies. Neurol Neuroimmunol Neuroinflamm. 2021; 8(2): e960. doi: 10.1212/NXI.0000000000000960. PMID: 33558306; PMCID: PMC8057062.
- Chard D, Trip SA. Resolving the clinico-radiological paradox in multiple sclerosis. F1000Res. 2017; 6: 1828. doi: 10.12688/f1000research.11932.1. PMID: 29093810; PMCID: PMC5645703.
- Solomon AJ, Arrambide G, Brownlee WJ, Flanagan EP, Amato MP, Amezcua L, et al. Differential diagnosis of suspected multiple sclerosis: an updated consensus approach. Lancet Neurol. 2023; 22(8): 750-768.
- Kabasawa H. MR Imaging in the 21st Century: Technical Innovation over the First Two Decades. Magn Reson Med Sci. 2022; 21(1): 71-82.
- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58(4): 512-521.