SciBase Journals
SciBase Neurology
ISSN 2996-3788
- Article Type: Research Article
- Volume 2, Issue 1
- Received: Feb 06, 2024
- Accepted: Mar 21, 2024
- Published Online: Mar 28, 2024
Changes in Immunoreactivity of Vimentin and GFAP in the Hippocampus of Patients with Drug-Resistant Epilepsy
Darya A Sitovskaya1,2*; Anastasiya O Eremina2 ; Tatyana V Sokolova1 ; Yulia M Zabrodskaya1,3
1Polenov Neurosurgical Institute, Branch of Almazov National Medical Research Centre, 191014, Mayakovskogo st., 12, SaintPetersburg, Russia.
2Department of Pathology with a Course of Forensic Medicine Named After D.D. Lochov, St. Petersburg State Pediatric Medical University, 194100, Litovskaya st., 2, Saint-Petersburg, Russia.
3The North-Western State Medical University Named After I.I. Mechnikov, 191015, Kirochnaia st., 41, Saint-Petersburg, Russia.
*Corresponding Author: Darya A Sitovskaya
Polenov Neurosurgical Institute, Branch of Almazov National Medical Research Centre, 191014, Mayakovskogo st. 12, Saint-Petersburg, Russia.
Email: daliya_16@mail.ru
Abstract
Epilepsy is a serious neurological disorder that can greatly impact a person’s quality of life and even result in disability. Unfortunately, despite the use of various antiepileptic drugs, more than 30% of patients develop a form of the disease that is resistant to medication, leaving surgery as the only treatment option. In order to better understand and potentially treat epilepsy, there has been a focus on the role of glial mechanisms and the potential for pathogenetic treatment. Specifically, researchers have looked at the expression of cytoskeletal proteins, such as Glial Fibrillary Acidic Protein (GFAP) and vimentin, in nervous tissue. Our study examined the expression of these proteins in the hippocampus of patients with different types of hippocampal sclerosis and Drug-Resistant Epilepsy (DRE). We found a significant increase in the expression of GFAP and vimentin in these structures, suggesting a potential role in the development of reactive gliosis. However, we did not find any correlations between these proteins in all areas studied. This suggests that the activation of these cytoskeletal proteins may occur independently and ultimately contribute to the development of reactive gliosis. These findings provide valuable insights into the mechanisms of epilepsy and may help improve diagnostic and treatment approaches.
Keywords: Drug-resistant epilepsy; Hippocampus; Vimentin; GFAP.
Citation: Sitovskaya DA, Eremina AO, Sokolova TV, Zabrodskaya YM. Changes in Immunoreactivity of Vimentin and GFAP in the Hippocampus of Patients with Drug-Resistant Epilepsy. SciBase Neurol. 2024; 2(1): 1012.
Introduction
Epilepsy is a severe neurological disorder that significantly impacts a person’s quality of life and can even lead to disability. Despite the availability of various antiepileptic drugs, more than 30% of patients develop a drug-resistant form of the disease, leaving surgical intervention as the only treatment option [1]. One of the causes of drug-resistant epilepsy is focal cortical dysplasia and hippocampal sclerosis, which are congenital abnormalities of brain development that result in the formation of pathological areas with giant neurons and abnormal astrocytes, typically in the frontal or temporal lobes. The diagnosis of this anomaly is crucial and requires the integration of immunohistochemical and genetic studies, as well as the possible incorporation of neuroimaging data into a multi-level system [2]. There is also a significant focus on the potential for pathogenetic treatment of epilepsy, particularly in relation to the expression of cytoskeletal proteins in nervous tissue, such as Glial Fibrillary Acidic Protein (GFAP) and vimentin, which are proteins of intermediate filaments type 3 [3]. These proteins often coexist in glial cell lines, particularly in astrocytes, regulating their activity. GFAP is necessary for fundamental cellular processes in astrocytes, such as motility, migration, and mitosis. By regulating the structure and functioning of astrocytes, GFAP influences the formation and plasticity of synapses, energy and redox metabolism, as well as the synaptic homeostasis of neurotransmitters and ions [4]. Vimentin increases the antigen-presenting function of astrocytes by influencing the vesicles within them [5]. It also promotes the inclusion of other proteins involved in the response of astrocytes to neurotrauma [6]. With a variety of etiological factors contributing to epilepsy, the pathogenesis is characterized by a disruption of the homeostasis of excitatory and inhibitory neurotransmitters in the neurons of the central nervous system. It is known that astrocytes play an important role in maintaining a constant environment in nervous tissue, and that dysfunction of these cells can contribute to the development of epilepsy [7,8]. Hippocampal sclerosis is a common condition found in temporal lobe epilepsy, characterized by changes in the cellular structure of the hippocampus, including neuronal death and gliosis [2]. While the term “sclerosis” typically refers to the compaction of an organ with the replacement of parenchymal cells by connective tissue, in the context of nervous system pathology, it also encompasses gliosis. According to the International League Against Epilepsy (ILAE) classification, one form of hippocampal sclerosis is characterized by gliosis alone, without any accompanying sclerosis. The role of vimentin and GFAP in regulating astrocytes suggests their potential involvement in epileptogenesis.
Purpose of the study
To examine the immunoreactivity of vimentin and GFAP in the hippocampal structures of patients with drug-resistant epilepsy
Materials and methods
The material of fragments of the temporal lobe and hippocampus of the Polenov Neurosurgical Institute – Branch of Almazov National Medical Research Centre, obtained intraoperatively from 30 patients with drug-resistant epilepsy, including 17 women, 13 men, average age 32.6 years. The comparison group was autopsy material from 10 patients who died from somatic diseases and had no history of neurological disorders (4 women, 6 men, average age 51 years). The protein content in tissue was studied using the Immunohistochemical (IHC) method according to standard methods. The following antibodies from Dako (CA, USA) were used for the study: mouse monoclonal anti-glial fibrillary acidic protein (M0761), 1:100; mouse monoclonal anti-vimentin (M0725), 1:200. The EnVision polymer detection system (Dako, CA, USA) was also used. For visualization, the streptavidin–peroxidase polymer ultrasensitive system and DAB chromogen (Sigma-Aldrich, Darmstadt, Germany) were used. The sections were counterstained with Gill’s hematoxylin and then embedded in Bio Mount HM synthetic embedding medium (BIO-OPTICA, Milano, Italy). Additionally, reactions lacking primary antibodies were carried outto ensure the specificity of the observed staining. Histological analysis and microphotography were performed using a Leica DM2500 M microscope equipped with a DFC320 digital camera and using an IM50 image manager (Leica Microsystems, Wetzlar, Germany). For IHC, positive staining with antibodies to GFAP and vimentin in sections of the temporal lobes was assessed by calculating the optical density (symb. units) of stained cells relative to the background areas in 10 fields of view at ×400 magnification using the Photo M 1.21 program. The result of the measurement is a coefficient obtained by dividing the density of positive areas with respect to the background. Statistical analysis was performed using Microsoft Office Excel 2010 (USA), Statistica v. 10. The Gaussianity of the sample was determined using the Kolmogorov–Smirnov test. Differences between two samples with measured attributes were determined using non-parametric statistical methods (for example, the Mann–Whitney U test if at least one of the samples was not normal).
Results
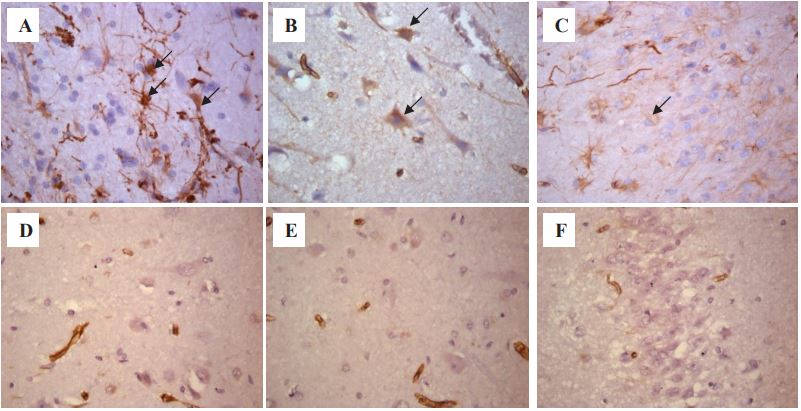
Histological examination revealed sclerosis of the hippocampus of various types in all patients. Type 1 hippocampal sclerosis was detected in 16 patients (53.3%), type 2 – in 2 patients (6.7%), type 3 – in 1 patient (3.3%), only gliosis without sclerosis – in 11 patients (36.7%). When examining the densitometric density of cells that were positively stained with antibodies to vimentin in the hippocampal formation of patients with DRE, the following results were obtained (Figure 1). IHC reactions with antibodies to vimentin, hippocampal structures in the CA1 nucleus were 0.019-0.048 (μ=0.028±0.006); in the CA4 nucleus 0.016-0.039 (μ=0.029±0.007); in the dentate gyrus 0.014–0.041 (μ=0.026±0.008). In patients in the comparison group, the densitometric density of vimentin in similar studied areas in the CA1 nucleus was 0.006-0.028 (μ=0.016±0.008); in the CA4 nucleus 0.006-0.02 (μ=0.014±0.005); in the dentate gyrus 0.008- 0.029 (μ=0.014±0.007).
A – CA1 nucleus of a patient with epilepsy. Reactive astrocytes are indicated by an arrow.
B – CA4 nucleus of a patient with epilepsy. Vimentin-positive neurons are indicated by an arrow.
C – Dentate gyrus of a patient with epilepsy. Vimentin-positive neurons of the granular layer are indicated by an arrow.
D – CA1 nucleus of the patient of the comparison group.
E – CA4 nucleus of the patient of the comparison group.
F – Dentate gyrus of the patient of the comparison group.
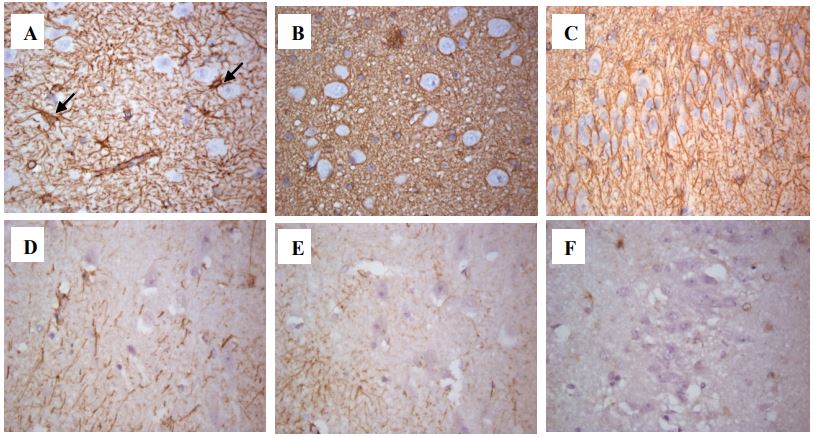
When examining the densitometric density of cells that were А B C positively stained with antibodies to GFAP in the hippocampal formation of patients with DRE, the following results were obtained (Figure 2). IHC reactions with antibodies to GFAP, hippocampal structures in the CA1 nucleus were 0.034-0.1 (μ=0.067±0.019); in the CA4 nucleus 0.037-0.121 (μ=0.078±0.021); in the dentate gyrus 0.028-0.268 (μ=0.087±0.052). In patients in the comparison group, the densitometric density of GFAP in similar studied areas in the CA1 core was 0.025-0.041 (μ=0.033±0.006); in the CA4 nucleus 0.007-0.037 (μ=0.022±0.011); in the dentate gyrus 0.019-0.038 (μ=0.026±0.007).
A – CA1 nucleus of a patient with epilepsy. Reactive astrocytes are indicated by an arrow.
B – CA4 nucleus of a patient with epilepsy.
C – Dentate gyrus of a patient with epilepsy.
D – CA1 nucleus of the patient of the comparison group.
E – CA4 nucleus of the patient of the comparison group.
F – Dentate gyrus of the patient of the comparison group.
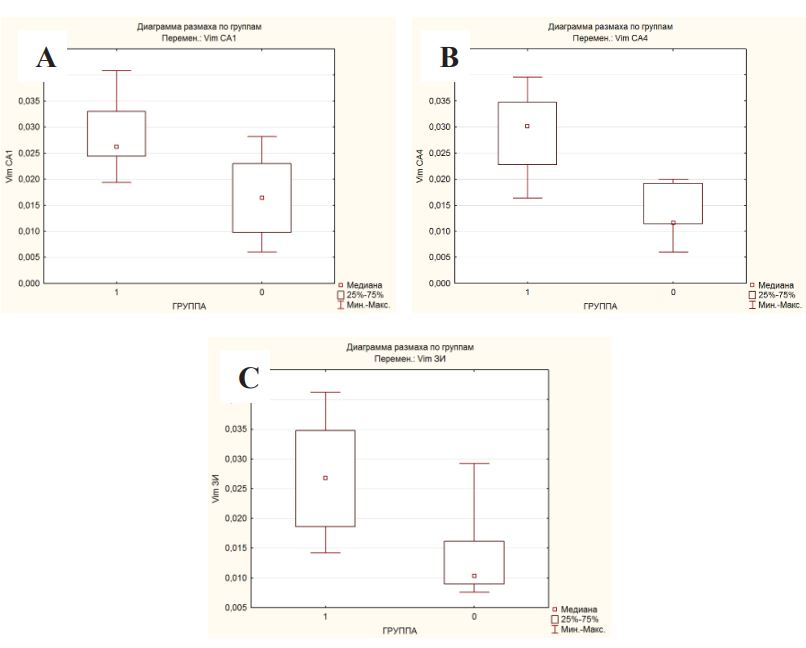
When statistically processing the data, a significant difference was obtained in the expression of vimentin in the hippocampus in patients with DRE and in the comparison group according to the Mann-Whitney U-test, Kolmogorov-Smirnov criteria (p<0.05) in all studied areas (Figures 3A-C; Table 1).
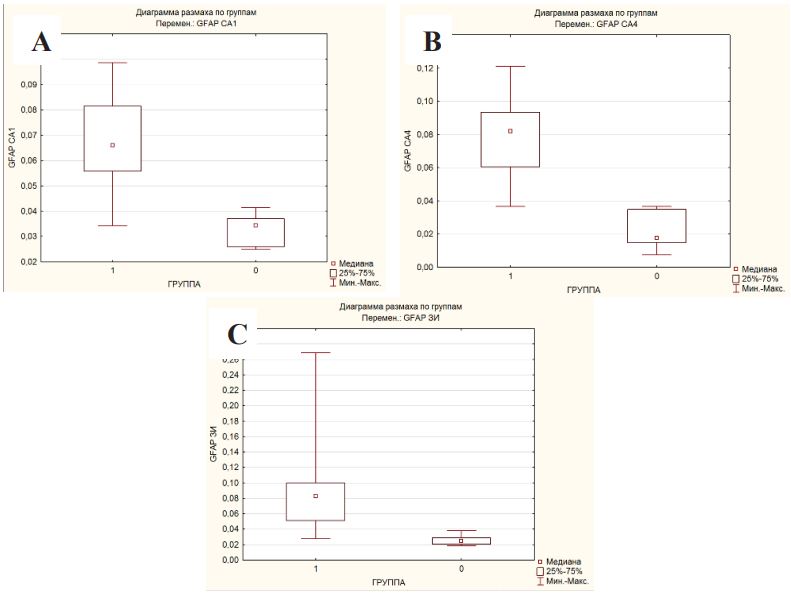
Table 1: Comparative characteristics of protein expression values in the hippocampus in patients with DRE and the comparison group (Mann-Whitney U-test).
| Hippocampus СА1 | |||
|---|---|---|---|
| Protein | Descriptive Statistics: μ | p-Value | |
| Patient with DRE | Comparison group | ||
| GFAP | 0,067 | 0,033 | 0,0004 |
| Vimentin | 0,028 | 0,016 | 0,0048 |
| Hippocampus СА4 | |||
| Protein | Descriptive Statistics: μ | p-Value | |
| Patient with DRE | Comparison group | ||
| GFAP | 0,078 | 0,022 | 0,0001 |
| Vimentin | 0,029 | 0,014 | 0,0012 |
| Hippocampus dentate gyrus | |||
| Protein | Descriptive Statistics: μ | p-Value | |
| Patient with DRE | Comparison group | ||
| GFAP | 0,087 | 0,026 | 0,0002 |
| Vimentin | 0,026 | 0,014 | 0,0048 |
Note: μ – the arithmetic mean; p-Value – the calculated level of significance.
When statistically processing the data, a significant difference was obtained in the expression of GFAP in the hippocampus in patients with DRE and in the comparison group according to the Mann-Whitney U-test, Kolmogorov-Smirnov criteria (p<0.05) in all studied areas (Figure 4A-C; Table 1).
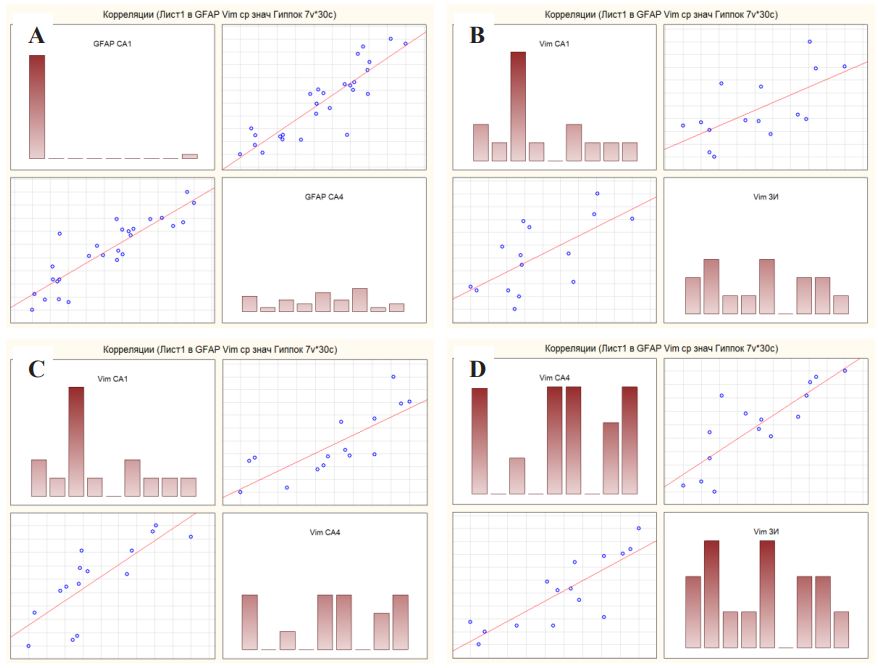
When studying the Spearman correlation coefficient between the studied proteins in the structures of the hippocampus, the following results were revealed. For GFAP expression in hippocampal nucleus CA1 and CA4, the Spearman correlation coefficient was 0.88, which corresponds to a high correlation strength on the Chaddock correlation coefficient scale (Figure 5A). For vimentin expression in hippocampal nucleus CA1 and CA4, the Spearman correlation coefficient was 0.9, which corresponds to a high correlation strength on the Chaddock correlation coefficient scale (Figure 5C). For vimentin expression in hippocampal nucleus CA1 and dentate gyrus, the Spearman correlation coefficient was 0.72, which corresponds to a high correlation strength on the Chaddock correlation coefficient scale (Figure 5B). For vimentin expression in hippocampal nucleus CA4 and dentate gyrus, the Spearman correlation coefficient was 0.83, which corresponds to a high correlation strength on the Chaddock correlation coefficient scale (Figure 5D). For other localizations, as well as between the proteins themselves, the correlations were very weak, which can be ignored. Also, no correlations were found between the level of protein expression and the type of hippocampal sclerosis, which can be explained by the small sample size.
Discussion
The most studied now is vimentin (OMIM 193060). Due to its extensive localization in the body (found in tissues of mesodermal origin), it performs many functions [9]. In the tissue of the Central Nervous System (CNS), the participation of vimentin in the development of neuroinflammation is important. It facilitates the transendothelial migration of lymphocytes across the blood-brain barrier [10], promotes the penetration of infectious agents into the endothelial cells of the CNS vessels [11], and is involved in the activation of microglia [12]. GFAP (OMIM 137780) is a protein that is one of the key elements of the cytoskeleton of astrocytes and is their characteristic marker. At least about ten GFAP isoforms have been identified [13], which can change the properties of astroglial cells. In the absence of GFAP, mice exhibited impaired myelin formation and organization of white matter in the brain [14], suggesting that this protein is required for the normal formation of these structures. The gene encoding GFAP is located on the long arm of chromosome [17,15], and its mutations are associated with a rare disease of the central nervous system – Alexander disease, the pathognomonic sign of which is the presence of Rosenthal fibers: protein aggregates in astrocytes containing GFAP. With this disease, there is a disturbance in the structure of the white matter with various neurological manifestations depending on the location of the lesion. Vimentin and GFAP are type 3 intermediate filament proteins. Due to their morphofunctional similarity, they can compensate each other. Thus, both compounds are involved in neurogenesis, while during the maturation of progenitor cells in astrocytes, a shift occurs in the predominance of intermediate filament proteins from vimentin to GFAP: vimentin is expressed predominantly in the brain tissue of newborns, and GFAP in the adult brain, since its protein product expression is characteristic of the late stages of astrocyte differentiation [16]. However, the exact age of onset of GFAP expression remains unclear. It is known that its primary appearance is observed in the bipolar cells of the ventricular zone, which are neuronal stem cells. In the middle of the early fetal period of intrauterine development, the subventricular zone becomes noticeable in the fetus, the neural progenitor cells of which also express GFAP, but this zone persists into adulthood, in contrast to the ventric ular zone, which disappears even before birth [17]. It has been shown that in late fetal forebrain fetuses, like adults, immunoreactivity to GFAP is found mainly in white matter astrocytes [18]. Currently, the role of vimentin and GFAP in the development of reactive gliosis is attracting much attention, since the increase in these markers, together with nestin and synemin in astrocytes, is a characteristic feature of astroglial activation in response to damage, ischemia, and neurodegeneration [19]. It was found that in mouse astrocytes with knockout of vimentin and GFAP, there is a lack of development of typical astrocyte hypertrophy, impaired formation of glial scars [20], as well as a slowdown in the immune response at the level of vesicular transport [5]. This suggests that vimentin and GFAP regulate the astroglial response to inflammation. Mice lacking vimentin and GFAP exhibited lower central nervous system resistance to mechanical stress [21] and ischemia [22]. Multiple factors indicate that vimentin and GFAP are important for the neuroprotective functions of astrocytes in acute ischemic stroke [9]. Moreover, astrogliosis of vimentin- and GFAP-immunoreactive astrocytes plays a positive role in the acute stages of the response to neurotrauma, but in later stages it limits the regenerative potential, worsens neurogenesis in the damaged area and promotes scar formation [20,23]. With a deficiency of vimentin and GFAP in reactive astrocytes during astrogliosis, better restoration of the damaged area of brain tissue is observed [20,23]. Regeneration after sciatic nerve injury in mice lacking vimentin and GFAP takes longer, but the result is the same [24]. The most recent study demonstrated that after brain injury, GFAP−/− Vim−/− mice exhibited maladaptive neuronal plasticity associated with impaired functional recovery, i.e. increased synaptic plasticity in the perilesional region and increased loss and gain of neural connections in sensorimotor networks [25]. This suggests that reactive astrocytes modulate plasticity responses that promote recovery after ischemic stroke. It should also not be excluded that with GFAP and vimentin deficiency, increased post-traumatic synaptic plasticity and increased basal and posttraumatic neurogenesis in the hippocampus are observed [26]. In our study, we found a significant increase in the expression of GFAP and vimentin in the structures of the hippocampus in patients with drug-resistant epilepsy; however, no correlations were found between these proteins in all studied areas. It can be assumed that the activation of these cytoskeletal proteins occurs independently and independently of each other, however, ultimately leads to the development of reactive gliosis.
Conclusions
The dysfunction of astrocytes, characterized by an increase in the expression of vimentin and GFAP, plays an important role in the development of epilepsy and the progression of neurodegeneration in affected patients. Further research on the mechanisms that contribute to this increase in expression is warranted, as it may lead to the identification of a potential therapeutic target for the treatment of epilepsy.
Declarations
Authors’ contributions
Study concept and design: Yu.M. Zabrodskaya, D.A. Sitovskaya.
Collection and processing of material: D.A. Sitovskaya, A.O. Eremina, T.V. Sokolova.
Statistical data processing: D.A. Sitovskaya, A.O. Eremina.
Text writing: D.A. Sitovskaya, A.O. Eremina.
Editing: D.A. Sitovskaya, Yu.M. Zabrodskaya.
Conflict of interest: The author declares no conflict of interest.
Financing: The study was carried out within the framework of State assignment No. 121031000359-3 «Development of new approaches in the diagnosis of mediobasal pharmacoresistant epilepsy based on the histoproteomics of epileptic foci».
Compliance with patient rights and principles of bioethics: All patients (or their representatives) gave written informed consent to participate in the study. The study was approved by the Ethics Committee of the Polenov Neurosurgical Institute – Branch of Almazov National Medical Research Centre St. Petersburg (protocol No. 0305-2016 of May 16, 2016) and was carried out in accordance with the Helsinki Declaration of Human Rights.
References
- Sone D. Making the Invisible Visible: Advanced Neuroimaging Techniques in Focal Epilepsy. Front Neurosci. 2021; 15: 699176. https://doi.org/10.3389/fnins.2021.699176. PMID: 34385902.
- Najm I, Lal D, Alonso Vanegas M, Cendes F, Lopes-Cendes I, Palmini A, Paglioli E, Sarnat HB, Walsh CA, Wiebe S, Aronica E, Baulac S, Coras R, Kobow K, Cross JH, Garbelli R, Holthausen H, Rössler K, Thom M, El-Osta A, Lee JH, Miyata H, Guerrini R, Piao YS, Zhou D, Blümcke I. The ILAE consensus classification of focal cortical dysplasia: An update proposed by an ad hoc task force of the ILAE diagnostic methods commission. Epilepsia. 2022; 63(8): 1899-1919. doi: 10.1111/epi.17301. Epub 2022 Jun 15. PMID: 35706131.
- Crespel A, Rigau V, Coubes P, Rousset MC, de Bock F, Okano H, Baldy-Moulinier M, Bockaert J, Lerner-Natoli M. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis. 2005; 19(3): 436-50. doi: 10.1016/j.nbd.2005.01.020. PMID: 16023586.
- Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011; 93(3): 421-43. doi: 10.1016/j.pneurobio.2011.01.005. Epub 2011 Jan 8. PMID: 21219963.
- Vardjan N, Gabrijel M, Potokar M, Svajger U, Kreft M, Jeras M, de Pablo Y, Faiz M, Pekny M, Zorec R. IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J Neuroinflammation. 2012; 9: 144. doi: 10.1186/1742-2094-9-144. PMID: 22734718; PMCID: PMC3423045.
- Jing R, Wilhelmsson U, Goodwill W, Li L, Pan Y, Pekny M, Skalli O. Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J Cell Sci. 2007; 120: 1267-77. doi: 10.1242/jcs.03423. Epub 2007 Mar 13. PMID: 17356066.
- Boison D, Steinhäuser C. Epilepsy and astrocyte energy metabolism. Glia. 2018; 66(6): 1235-1243. doi: 10.1002/glia.23247. Epub 2017 Oct 17. PMID: 29044647; PMCID: PMC5903956.
- Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016; 131(3): 323-45. doi: 10.1007/s00401-015-1513-1. Epub 2015 Dec 15. PMID: 26671410.
- Ridge KM, Eriksson JE, Pekny M, Goldman RD. Roles of vimentin in health and disease. Genes Dev. 2022; 36(7-8): 391-407. doi: 10.1101/gad.349358.122. PMID: 35487686; PMCID: PMC9067405.
- Bayir E, Sendemir A. Role of Intermediate Filaments in BloodBrain Barrier in Health and Disease. Cells. 2021; 10(6): 1400. doi: 10.3390/cells10061400. PMID: 34198868; PMCID: PMC8226756.
- Villarreal R, Manzer HS, Keestra-Gounder AM, Doran KS. Vimentin Regulates Chemokine Expression and NOD2 Activation in Brain Endothelium during Group B Streptococcal Infection. Infect Immun. 2021; 89(12): e0034021. doi: 10.1128/IAI.00340-21. Epub 2021 Sep 7. PMID: 34491787; PMCID: PMC8594594.
- Jiang SX, Slinn J, Aylsworth A, Hou ST. Vimentin participates in microglia activation and neurotoxicity in cerebral ischemia. J Neurochem. 2012; 122(4): 764-74. doi: 10.1111/j.1471-4159.2012. 07823.x. Epub 2012 Jun. 27. Erratum in: J Neurochem. 2021; 158(2): 571-572. PMID: 22681613.
- Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015; 32: 121-30. doi: 10.1016/j.ceb.2015.02.004. Epub 2015 Mar 2. PMID: 25726916.
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996; 17(4): 607-15. doi: 10.1016/s0896- 6273(00)80194-4. PMID: 8893019.
- Brownell E, Lee AS, Pekar SK, Pravtcheva D, Ruddle FH, Bayney RM. Glial fibrillary acid protein, an astrocytic-specific marker, maps to human chromosome 17. Genomics. 1991; 10(4): 1087-9. doi: 10.1016/0888-7543(91)90205-s. PMID: 1655631.
- Dahl D. The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. J Neurosci Res. 1981; 6(6): 741-8. doi: 10.1002/jnr.490060608. PMID: 7334533.
- Tramontin AD, García-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003; 13(6): 580-7. doi: 10.1093/cercor/13.6.580. PMID: 12764031.
- Kharlamova, A.S. Characteristics of gliogenesis in the human prepiriform cortex in early fetal ontogenesis / A. S. Kharlamova, O. S. Godovalova // Clinical and experimental morphology. – 2018; (25): 34-41. [Harlamova, A. S. Harakteristika gliogeneza v prepiriformnoj kore cheloveka v rannem fetal’nom ontogeneze / A. S. Harlamova, O. S. Godovalova // Klinicheskaja i jeksperimental’naja morfologija. – 2018. – No. 1(25). – S. 34-41].
- Bayir E, Sendemir A. Role of Intermediate Filaments in BloodBrain Barrier in Health and Disease. Cells. 2021; 10(6): 1400. doi: 10.3390/cells10061400. PMID: 34198868; PMCID: PMC8226756.
- Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004; 24(21): 5016-21. doi: 10.1523/JNEUROSCI.0820-04.2004. PMID: 15163694; PMCID: PMC6729371.
- Verardo MR, Lewis GP, Takeda M, Linberg KA, Byun J, Luna G, Wilhelmsson U, Pekny M, Chen DF, Fisher SK. Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest Ophthalmol Vis Sci. 2008; 49(8): 3659-65. doi: 10.1167/iovs.07-1474. Epub 2008 May 9. PMID: 18469190; PMCID: PMC2650509.
- De Pablo Y, Nilsson M, Pekna M, Pekny M. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochem Cell Biol. 2013; 140(1): 81-91. doi: 10.1007/s00418-013-1110-0. Epub 2013 Jun 12. PMID: 23756782.
- Pekny M, Wilhelmsson U, Tatlisumak T, Pekna M. Astrocyte activation and reactive gliosis-A new target in stroke? Neurosci Lett. 2019; 689: 45-55. doi: 10.1016/j.neulet.2018.07.021. Epub 2018 Jul 17. PMID: 30025833.
- Berg A, Zelano J, Pekna M, Wilhelmsson U, Pekny M, Cullheim S. Axonal regeneration after sciatic nerve lesion is delayed but complete in GFAP- and vimentin-deficient mice. PLoS One. 2013; 8(11): 79395. doi: 10.1371/journal.pone.0079395. PMID: 24223940; PMCID: PMC3815133.
- Aswendt M, Wilhelmsson U, Wieters F, Stokowska A, Schmitt FJ, Pallast N, de Pablo Y, Mohammed L, Hoehn M, Pekna M, Pekny M. Reactive astrocytes prevent maladaptive plasticity after ischemic stroke. Prog Neurobiol. 2022; 209: 102199. doi: 10.1016/j.pneurobio.2021.102199. Epub 2021 Dec 15. PMID: 34921928.
- Wilhelmsson U, Pozo-Rodrigalvarez A, Kalm M, de Pablo Y, Widestrand Å, Pekna M, Pekny M. The role of GFAP and vimentin in learning and memory. Biol Chem. 2019; 400(9): 1147-1156. doi: 10.1515/hsz-2019-0199. PMID: 31063456.