SciBase Journals
SciBase Neurology
ISSN 2691-7785
- Article Type: Case Report
- Volume 2, Issue 1
- Received: Apr 01, 2024
- Accepted: May 17, 2024
- Published Online: May 24, 2024
Chance Encounter of Mega Cisterna Magna in a Case with Cerebellar Diaschisis following Ischemic Stroke
Hsiang-En Tsai1,2; Tsai-Yun Wu3 ; Shin-Tsu Chang4,5*
1School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2Department of Medical Education, Chung Shan Medical University Hospital, Taichung, Taiwan.
3Graduate Institute of Biochemistry and Molecular Biology, College of Medicine, National Taiwan University, Taipei, Taiwan.
4Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
5Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan.
*Corresponding Author: Shin-Tsu Chang
Department of physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
Tel: 886-935-605578;
Email: ccdivlaser1959@gmail.com
Abstract
Mega Cisterna Magna (MCM) has been known as a developmental abnormality within the Dandy-Walker complex, characterized by an expanded cisterna magna exceeding 10 mm. While MCM often presents asymptomatically, it has been associated with mental disorders and cognitive decline. Differential diagnosis between MCM and arachnoid cyst is crucial due to their shared embryonic origin. Crossed Cerebellar Diaschisis (CCD) denotes functional decline in the contralateral cerebellar hemisphere due to a supratentorial lesion. CCD has associations with postural disturbance and complex regional pain syndrome. Notably, no literature discusses the co-occurrence of MCM and CCD. Here, we present a unique case involving underscore the significance and rarity of the change encounter of MCM and CCD. To our knowledge, this is the first case linking CCD to MCM, highlighting the need for further research elucidating their relationship and impact on clinical outcomes.
Keywords: Mega cisterna magna; Crossed cerebellar diaschisis; Change encounter; Ischemic stroke; Scintigraphic rehabilitation; Single photon emission computed tomography; Arachnoid cyst.
Citation: Tsai HE, Wu TY, Chang ST. Chance Encounter of Mega Cisterna Magna in a Case with Cerebellar Diaschisis following Ischemic Stroke. SciBase Neurol. 2024; 2(1): 1014.
Introduction
Mega Cisterna Magna (MCM) represents a subtype of the Dandy-Walker complex, characterized by an expanded cisterna magna exceeding 10 mm, situated in the inferior and posterior fossa CSF filling space. The MCM is hypothesized to result from the late fenestration of Blake’s pouch during embryonic development [1]. Its prevalence is estimated at approximately 2.5 per 1000 in a study encompassing 19,301 consecutive Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) scans of the brain conducted in northern Israel [2]. While often asymptomatic, MCM has been associated with psychotic and cognitive disorders [3-6].Crossed Cerebellar Diaschisis (CCD) manifests as decreased metabolism/blood flow in the contralateral cerebellar hemisphere secondary to a supratentorial cerebral lesion/injury, diagnosed through positron-emission tomography (PET) or Single-Photon Emission CT (SPECT) [7]. Typically asymptomatic, CCD is considered a negative prognostic factor and can contribute to postural disturbance [8].
However, the co-occurrence of MCM and CCD has never been reported. Therefore, we present a case illustrating the simultaneous presence of MCM and CCD in a patient at a medical center in Taiwan.
Case presentation
A 62-year-old female with a history of both poorly controlled hypertension and diabetes mellitus due to non-adherence presented with a cold and intermittent cough since April 2023. However, her family reported that she had been experiencing difficulties maintaining an upright position, along with symptoms of malaise, neglect, excessive sleepiness, and loss of appetite since the morning of May 11th.
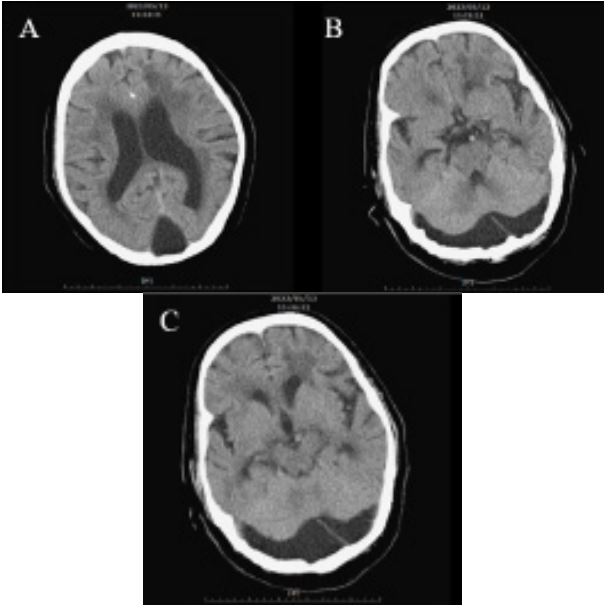
Subsequently, she was brought to our emergency department for assistance on May 13th. Upon triage, her vital signs indicated a systolic blood pressure of 262 mmHg, a Glasgow Coma Scale score of E4M6V1, and a bedridden status. X-rays revealed bilateral pulmonary edema, while a noncontrast CT scan of the brain revealed no evidence of hemorrhage but did reveal the presence of a MCM (Figure 1). Following a consultation with the neurology team, the patient was prescribed Aspirin with Dipyridamole for secondary prevention, Mannitol 300 CC IV stat, and 150 CC IV Q12H for brain edema. She was subsequently referred to the neurology ward for further management.
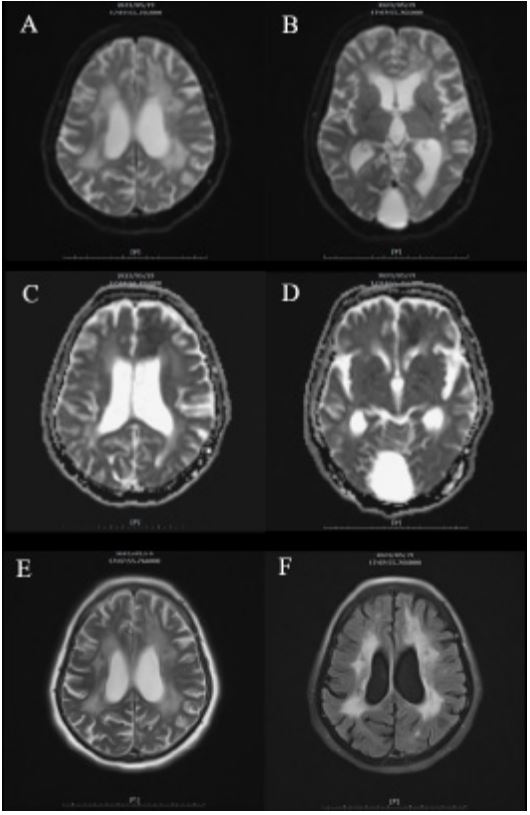
Upon admission on May 14th, the patient exhibited dysarthria, right central palsy, and right hemiplegia (0/5 strength in the right upper limb and 0/5 in the right lower limb), with a Babinski sign presenting on the right side. MRI findings revealed recent infarction characterized by high signal intensities on T2- Weighted Imaging (T2WI), FLAIR images, and diffusion-weighted images, along with a lower apparent diffusion coefficient value, localized in the deep white matter of the left frontal lobe and the left aspect of the corpus callosum (Figure 2). This was attributed to stenosis in the left M1 segment of the left middle cerebral artery. Her modified Rankin score was assessed as 4/6. Additionally, the presence of severe periventricular high signal intensities on T2WI and FLAIR images suggested a possible diagnosis of subcortical arteriosclerotic encephalopathy, commonly known as Binswanger disease. A Rehabilitation (REH) consultation was initiated on May 23rd. The patient demonstrated moderate assistance in bed rolling and transitioning from supine to sitting, and maximum dependence when attempting to stand.
Despite maintaining continent bladder control, her poor ambulation and balance led to maximal dependence in activities of daily living. Consequently, bedside occupational therapy and physical therapy were recommended.
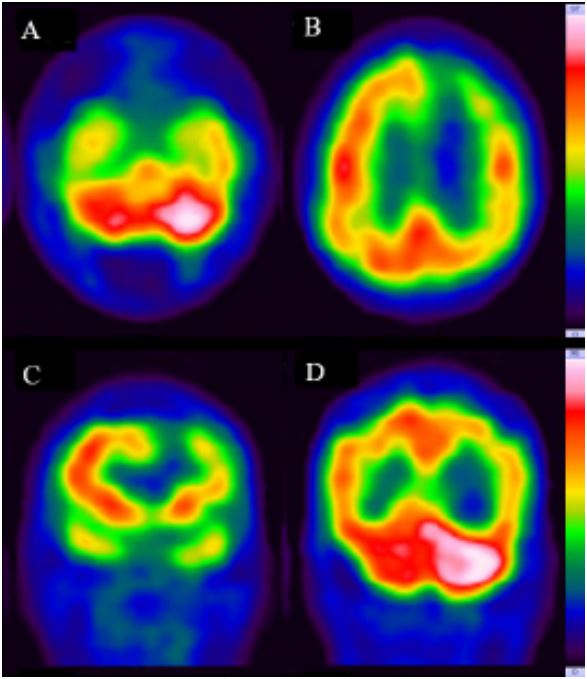
However, due to the progression of pneumonia and intermittent episodes of desaturation, the patient remained in the neurology ward for treatment until her clinical condition stabilized on July 22nd. Subsequently, she was referred to our REH outpatient clinic on account of persistent left hemiplegia as of August 14th. A cerebral blood flow was conducted by the 99mTc-Ethyl Cysteinate Dimer (ECD) SPECT/CT to assess the decline in cerebral function across different regions, which images indicated prior damage in the left frontal and occipital regions, as well as the left basal ganglion/thalamus. Additionally, evidence of penumbra in the left cerebral hemisphere and Binswanger disease in the periventricular white matter was observed. Furthermore, the presence of crossed cerebellar diaschisis was noted due to reduced blood flow in the right cerebellar hemisphere (Figure 3). Consequently, an intensive rehabilitation therapy was initiated. Muscle stimulation and low-power laser therapy were also prescribed to address the right-sided hemiplegia. The patient’s rehabilitation program is currently ongoing.
Discussion
We present a case detailing the change encounter of MCM concomitant with right-sided CCD following a left M1 ischemic stroke. The presence of MCM was confirmed through CT and MRI imaging, while CCD was identified via SPECT imaging. Given the rarity of co-occurrence between MCM and CCD, we aim to elucidate this unique case by compiling relevant literature and data.
Cisterna magna, characterized by focal enlargement of the Cerebral Spinal Fluid (CSF)-filled subarachnoid space, is situated in the posterior fossa dorsal to the medulla and caudal to the cerebellum, with a prevalence of approximately 2.5 per 1000 individuals [2]. MCM denotes an expansion of the subarachnoid space within the cisterna magna to 10 mm in the posterior fossa, occasionally accompanied by scalloping of the occipital inner table, often attributed to leptomeningeal underdevelopment [9,10]. MCM represents a subtype within the Dandy-Walker complex, with the other three subtypes being Dandy-Walker malformation, Dandy-Walker variation, and subarachnoid cyst [11]. The prevalence of MCM has been reported to be approximately 1 in 8268 individuals in a study of a 3 million population, as documented by regional survey and associated databases spanning from 1995 to 2004 [12]. While MCM is often asymptomatic and typically does not necessitate treatment due to its stable clinical presentation [11], differential diagnosis from arachnoid cysts, particularly the retrocerebellar type, is imperative [13]. Nevertheless, MCM has been associated with an increased incidence of psychological disorders such as schizophrenia, bipolar disorder, mania, and catatonia, and may contribute to cognitive decline, affecting executive function, memory, and verbal ability [3-6].
A meta-analysis involving 531 fetuses revealed rates of additional anomalies in the Central Nervous System (CNS) and extra-CNS of 12.6% and 16.6%, respectively, among 144 fetuses. Additionally, ventriculomegaly was identified in 11.7% of cases, making it the most common associated anomaly [14]. Moreover, MCM may alter various cerebral cortical functions by modulating cortical activity throughout the cortico-cerebellarthalamic-cortical circuitry [15].
Arachnoid cysts represent a subtype within the Dandy-Walker complex and account for at least 1% of all intracranial mass lesions [16]. A cross-sectional study involving 48,417 patients demonstrated that arachnoid cysts are predominantly located in the middle fossa (34%), retrocerebellar region (33.3%), and convexity (14%) [17]. While small arachnoid cysts are typically asymptomatic, larger cysts may cause symptoms such as headache, nausea, vomiting, and dizziness due to mass effects. Seizures, ataxia, and emotional changes may also manifest in cases with severe intracranial defects [18]. The presence of mass effect serves as a distinguishing feature from MCM, presenting as mid-line shift and compression to the vermis, cerebellar hemispheres, and brainstem, leading to the aforementioned symptoms [19,20]. However, in our case, the presence of free falx cerebri and insufficent compression signs implies no mass effect, leading us to consider MCM rather than arachnoid cyst in our diagnostic deliberations (Figure 1).
In our case, hyper-intensity lesions within the bilateral periventricular white matter suggest focal characteristics associated with Binswanger’s Disease (BD). BD is recognized as a subgroup of cerebral small vessel disease primarily affecting the cerebral white matter [21]. Common risk factors comprise hypertension, diabetes mellitus, dyslipidemia, and cigarette exposure. Clinical features encompass cognitive impairment, upper motor neuron signs, hypertension, and progressive symptoms. MRI and CT imaging play crucial roles in diagnosing BD. Although periventricular white matter degeneration is strongly associated with aging, the classic radiological features of BD include hyper-intensity and bilateral symmetric signals among the periventricular white matter, frontal horns, and anterior frontal lobe in T2 and FLAIR sequences, with corresponding hypo-dense lesions on CT imaging. Notably, the presence of ascending lesions extending from the periventricular white matter to the corona radiata represents a specific and crucial characteristic of BD [22-24]. Although our case exhibits risk factors such as hypertension and diabetes mellitus, along with pyramidal tract symptoms and cognitive degeneration, the absence of ascending lesions raises the need to consider other causes of white matter hyperintensities or leukoaraiosis. Nevertheless, we maintain a high suspicion for the diagnosis of Binswanger’s disease in our case, based on the clinical patterns and correlation with imaging findings.
Crossed Cerebellar Diaschisis (CCD) refers to a decline in functional activity, metabolism, and perfusion in the contralateral cerebellar hemisphere due to a supratentorial lesion. The mechanism of CCD is often attributed to damage to the cortico-ponto-cerebellar pathway resulting from ischemic or hemorrhagic stroke [25]. Other pathways, such as the corticorubral pathway and the bidirectionality of the dentato-rubrothalamo-cortical tract, are also implicated [26,27]. While the initial presentations of functional CCD are typically elusive, structural CCD, which affects volume reduction in lobule VI, has been proven to be related to motor dysfunction and severity of disability [28]. CCD on the left side has also been associated with poor trunk control performance, evidenced by increased sway intensity and velocity [8]. Moreover, CCD has been linked to postural disturbance, ipsilateral and contralateral thalamic diaschisis, complex regional pain syndrome, glenohumeral subluxation, and parakinesia brachialis oscitans [8,29-32].
To our knowledge, this is the first case of incidental finding of MCM with CCD after acute ischemic stroke. It seems reasonable to consider CCD as an additional CNS problem associated with MCM due to anatomical abnormalities. Additionally, CCD is considered a negative functional prognostic factor, whether after acute or chronic stroke, affecting motor function and cerebral perfusion performance [8,31,33-35]. Meanwhile, MCM may utilize the cortico-cerebellar-thalamic-cortical circuit to alter cerebral cortical function and potentially interfere with disease performance in patients [15]. Consequently, it may be reasonable to hypothesize that severe postural imbalance with moderate cognitive impairment may also be linked to congenital MCM interruption, particularly in cases such as ours with right-sided CCD, which may not significantly affect gait. However, due to limited research, we can only speculate on the unique phenomenon of the coexistence of MCM and CCD. The relationship between MCM and CCD, as well as their actual effects on patient physical function, remains unknown.
In our case, 99mTc-ECD-SPECT/CT played a crucial role in diagnosing CCD, which brain images have been utilized across various spectra in scintigraphic rehabilitation to detect functional lesions and aid in further differential diagnosis, encompassing conditions such as CCD, ipsilateral cerebellar diaschisis, ipsilateral thalamic diaschisis, brain tumors and their mimics, dementia, and epilepsy [8,36,37]. With its ability to observe objective brain blood flow or utilize specific nuclear medicine markers such as I123 Iomazenil, I123 Iofupane, and Tl201, the significant contribution of SPECT cannot be overstated [36,38].
It is crucial to acknowledge the limitations of the current study. Firstly, as the patient was not admitted to our hospital prior to the stroke episode, we were unable to establish baseline assessments of mental, cognitive, and motor function. Consequently, distinguishing between the patient’s clinical presentation as indicative of their baseline status or as a consequence of the disease became challenging. Secondly, due to the rarity of the condition, we lacked a sufficient control group to fully assess the impact of MCM with CCD. Thirdly, the limited availability of data and literature prevented us from elucidating the relationship between MCM and CCD, as well as their respective influences on the patient’s clinical condition.
Conclusion
Our current case presentation represents the first instance connecting CCD to MCM and underscores the rarity of their change encounter. Future research endeavors with larger-scale studies are warranted to ascertain the influence of MCM on CCD. Brain SPECT plays an important role in the discipline of scintigraphic rehabilitation.
References
- Kau T, Marterer R, Kottke R, et al. Blake’s Pouch Cysts and Differential Diagnoses in Prenatal and Postnatal MRI : A Pictorial Review. Clin Neuroradiol. 2020; 30(3): 435-445.
- Zimmer EZ, Lowenstein L, Bronshtein M, et al. Clinical Significance of Isolated Mega Cisterna magna. Arch Gynecol Obstet. 2007; 276(5): 487-490.
- Yazici E, Kose S, Gunduz Y, Kurt EM, Yazici AB. Mega Cisterna Magna in Bipolar Mood Disorder: A Case Report. J Yeungnam Med Sci. 2022; 39(1): 58-61.
- Pandurangi S, Pandurangi A, Matkar A, Shetty N, Patil P. Psychiatric Manifestations Associated with Mega Cisterna Magna. J Neuropsychiatry Clin Neurosci. 2014; 26(2): 169-171.
- Kumar S, Sur S, Singh A. Mega Cisterna Magna Associated with Recurrent Catatonia: A Case Report. Biol Psychiatry. 2011; 70(4): e19.
- Turan T, Beşirli A, Asdemir A, Ozsoy S, Eşel E. Manic Episode Associated with Mega Cisterna Magna. Psychiatry Investig. 2010; 7(4): 305-307.
- Lin DD, Kleinman JT, Wityk RJ, et al. Crossed Cerebellar Diaschisis in Acute Stroke Detected by Dynamic Susceptibility Contrast MR Perfusion Imaging. AJNR Am J Neuroradiol. 2009; 30(4): 710-715.
- Chang CC, Ku CH, Chang ST. Postural Asymmetry Correlated with Lateralization of Cerebellar Perfusion in Persons with Chronic Stroke: A Role of Crossed Cerebellar Diaschisis in Left Side. Brain Inj. 2017; 31(1): 90-97.
- Chaudhari BP, Ho ML. Congenital Brain Malformations: An Integrated Diagnostic Approach. Semin Pediatr Neurol. 2022; 42: 100973.
- Bosemani T, Orman G, Boltshauser E, Tekes A, Huisman TA, Poretti A. Congenital Abnormalities of the Posterior fossa. Radiographics. 2015; 35(1): 200-220.
- Bansal A, Reddy GS, Chug A, Damaraju SC. Massive Posterior Cranial Vault Erosion and Its Reconstruction: A Peculiar Presentation of Mega Cisterna Magna. J Oral Biol Craniofac Res. 2021; 11(1): 13-16.
- Long A, Moran P, Robson S. Outcome of Fetal Cerebral Posterior Fossa Anomalies. Prenat Diagn. 2006; 26(8): 707-710.
- Correa GG, Amaral LF, Vedolin LM. Neuroimaging of DandyWalker Malformation: New Concepts. Top Magn Reson Imaging. 2011; 22(6): 303-312.
- D’Antonio F, Khalil A, Garel C, et al. Systematic Review and MetaAnalysis of Isolated Posterior Fossa Malformations on Prenatal Ultrasound Imaging (part 1): Nomenclature, Diagnostic Accuracy and Associated Anomalies. Ultrasound Obstet Gynecol. 2016; 47(6): 690-697.
- Andreasen NC, Pierson R. The Role of the Cerebellum in Schizophrenia. Biol Psychiatry. 2008; 64(2): 81-88.
- Vega-Sosa A, de Obieta-Cruz E, Hernández-Rojas MA. Intracranial Arachnoid Cyst. Cir Cir. 2010; 78(6): 551-556.
- Al-Holou WN, Terman S, Kilburg C, Garton HJ, Muraszko KM, et al. Prevalence and Natural History of Arachnoid Cysts in Adults. J Neurosurg. 2013; 118(2): 222-231.
- Wojcik G. WiadLek. 2016; 69(3 pt 2): 555-559.
- Shekdar K. Posterior Fossa Malformations. Semin Ultrasound CT MR. 2011; 32(3): 228-241.
- Shettar M, Karkal R, Misra R, Kakunje A, Mohan Chandran VV, et al. Arachnoid Cyst Causing Depression and Neuropsychiatric Symptoms: a Case Report. East Asian Arch Psychiatry. 2018; 28(2): 64-67.
- Litak J, Mazurek M, Kulesza B, et al. Cerebral Small Vessel Disease. Int J Mol Sci. 2020; 21(24): 9729.
- Huisa BN, Rosenberg GA. Binswanger’s Disease: Toward a Diagnosis Agreement and Therapeutic Approach. Expert Rev Neurother. 2014; 14(10): 1203-1213.
- Văcăraș V, Cordoș AM, Rahovan I, Frunze S, Mureșanu DF. Binswanger’s Disease: Case Presentation and Differential Diagnosis. Clin Case Rep. 2020; 8(12): 3450-3457.
- Caplan LR, Gomes JA. Binswanger Disease--An Update. J Neurol Sci. 2010; 299(1-2): 9-10.
- Sommer WH, Bollwein C, Thierfelder KM, et al. Crossed Cerebellar Diaschisis in Patients with Acute Middle Cerebral Artery Infarction: Occurrence and Perfusion Characteristics. J Cereb Blood Flow Metab. 2016; 36(4): 743-754.
- Zhu Y, Ruan G, Cheng Z, Zou S, Zhu X. Lateralization of the Crossed Cerebellar Diaschisis-Associated Metabolic Connectivities in Cortico-Ponto-Cerebellar and Cortico-Rubral Pathways. Neuroimage. 2022; 260: 119487.
- He HC, Hsu MC, Hsu CS, Cheng YY, Chang ST. Bidirectionality of the Dentato-Rubro-Thalamo-Cortical Tract Allows Concurrent Hypoperfusion in Ipsilateral Cerebellum and Contralateral Cerebral Hemisphere: A Case Report. Medicine (Baltimore). 2018; 97(40): e12590.
- Guder S, Sadeghi F, Zittel S, et al. Disability and Persistent Motor Deficits are Linked to Structural Crossed Cerebellar Diaschisis in Chronic Stroke. Hum Brain Mapp. 2023; 44(16): 5336-5345.
- Wang KC, Wu YC, Chao WH, et al. Appearance of Ipsilateral and Contralateral Thalamic Diaschisis Together with Crossed Cerebellar Diaschisis Viewed From Brain Perfusion Images In A Case with Acute Ischemic Stroke, International Journal of Multidisciplinary Research and Growth Evaluation. 2022; 3(2): 170-174.
- Lai MH, Wang TY, Chang CC, Li TY, Chang ST. Cerebellar Diaschisis and Contralateral Thalamus Hyperperfusion in a Stroke Patient with Complex Regional Pain Syndrome. J Clin Neurosci. 2008; 15(10): 1166-1168.
- Hsieh TY, Liu CC, He HC, Cheng YY, Chang ST. Persistence of Glenohumeral Subluxation is Correlated with Prolonged Existence of Crossed Cerebellar Diaschisis in a Hemiplegic Stroke Survivor: A Pilot Study. Gerontol. Geriatr. Res. 2020; 2: 1-6.
- Wu YT, Chang ST, Chen LC, Li TY. Concurrence of Crossed Cerebellar Diaschisis and Parakinesia Brachialis Oscitans in a Patient with Hemorrhagic Stroke. Case Rep Med. 2013; 2013: 519808.
- Kunz WG, Sommer WH, Höhne C, et al. Crossed Cerebellar Diaschisis in Acute Ischemic Stroke: Impact on Morphologic and Functional Outcome. J Cereb Blood Flow Metab. 2017; 37(11): 3615-3624.
- Wu C, Jiang B, Sun L, Hu T, Zhang Z, et al. A Subtle Connection between Crossed Cerebellar Diaschisis and Supratentorial Collateral Circulation in Subacute and Chronic Ischemic Stroke. J Stroke Cerebrovasc Dis. 2022; 31(12): 106856.
- Chen W, He S, Song H, et al. Quantitative Ischemic Characteristics and Prognostic Analysis of Crossed Cerebellar Diaschisis in Hyperacute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2022; 31(4): 106344.
- Davis KM, Ryan JL, Aaron VD, Sims JB. PET and SPECT Imaging of the Brain: History, Technical Considerations, Applications, and Radiotracers. Semin Ultrasound CT MR. 2020; 41(6): 521-529.
- Chu YC, Chang ST, Chan HY, Shen DHY, Chan HP. Abnormalities in Regional Cerebral Blood Flow Due to Headache in a COVID-19 Infected Patient Observed on 99mTC-ECD Brain SPECT/CT. Reports. 2023; 6(4): 58.
- Kaneta T. PET and SPECT Imaging of the Brain: A Review on the Current Status of Nuclear Medicine in Japan. Jpn J Radiol. 2020; 38(4): 343-357.