SciBase Journals
SciBase Oncology
ISSN 2691-7785
- Article Type: Research Article
- Volume 1, Issue 1
- Received: Aug 22, 2023
- Accepted: Oct 06, 2023
- Published Online: Oct 13, 2023
Methotrexate-Loaded PLGA Nanoparticle: Extended Survival of GBM-Bearing Rats
Fatemeh Madani; Masood Khosravani*; Mahdi Adabi*
Department of Medical Nanotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
*Corresponding Author: Khosravani M & Adabi M
Department of Medical Nanotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Tel: +98-21-43052132;
Email: drkhosravani@tums.ac.ir & madabi@tums.ac.ir
Abstract
This research expresses the preparation and investigation of methotrexate-loaded PLGA nanoparticles on Glioblastoma Multiforme (GBM). The nanoparticles were prepared by precipitation method which had a mean diameter of 102±5 nm with a zeta potential of -26±2 mV. The methotrexate loading was 8.3%. The results of the MTT assay demonstrated that 56% of C6 cells were viable when encountering methotrexate-loaded PLGA nanoparticles for 48 h. In-vivo assessments showed that the tumor size of GBM-bearing rats was partially increased after 28 days and moreover, the mean survival time of rats extended to 31 days.
Keywords: Methotrexate; PLGA; PVA; Glioblastoma; Nanoparticle.
Citation: Madani F, Khosravani M, Adabi M. Methotrexate-Loaded PLGA Nanoparticle: Extended Survival of GBM-Bearing Rats. SciBase Oncol. 2023; 1(1): 1003.
Introduction
GBM is known as the highest prevalence primary brain tumor with a number of 3.21 per 100,000 cases, which is a companion with a poor prognosis that is 5.5% for more than 5 years [1]. The treatment process commences with surgery, followed by chemotherapy and tumor treatment. Even though the therapy measurements are upgraded, the survival of patients is still a skimp and it reveals that novel strategies for total treatment of the GBM are highly in demand. Besides the mentioned challenges, tumor recurrence (≈90%) and expensive treatments made the GBM patients encounter intricate situations [2].
One of the problems in the treatment of this disease is that most chemotherapy drugs are not able to reach the brain because of the Blood Brain Barrier (BBB). It means that there is a complex structure of cerebral capillaries, which have the property of selective permeability, and do not allow any unknown substance to pass through [3-5]. The barrier is made up of endothelial cells, astrocytes, pericytes and tight junction proteins that keep the endothelial cells in order and next to each other [6]. Another problem in drug delivery to the tumor is the efflux pumps that expel the drugs after entering the cell [7]. Various types of nanoparticles from organic to inorganic materials are introduced and are under research with the aim of improving the treatment process [8-10]. One approach is to deliver chemotherapy drugs to the tumor by nanoparticles [11,12]. Among nanoparticles, polymerics are very popular and divided into natural, synthetic, and semi-synthetic subgroups. PLGA is from synthetic ones and PLGA-based nanostructures are under wide studies because of their extraordinary features such as sustained drug release, biocompatibility, biodegradability, ease of surface modification with hydrophilic agents, and ability to load both hydrophilic and hydrophobic substances [13]. There are various studies that have loaded chemotherapeutics in PLGA nanoparticles with the aim of GBM treatments [14-16].
Methotrexate is an anti-inflammatory and anticancer agent that is the antagonist of folic acid but its application has been shrunk due to toxicity-related complications [17]. The side effects of methotrexate encompass a wide range from non-significant gastrointestinal disorders such as decrement of appetite, nausea, and vomiting to serious problems like hepatic failure [18]. The effectiveness of methotrexate on GBM cell lines was observed in several researches however the challenge is that methotrexate is not able to permeate via BBB, properly [19-21].
In the current study we intended to evaluate the efficacy of methotrexate-loaded PLGA on the GBM animal model.
Materials and methods
Methotrexate and PLGA (50:50) were purchased from Xi’an Xinlu Biotech Company (China) and acetone was bought from Dr. Mojallali’s Industrial Chemical Complex Company (Iran). PVA was obtained from Merck (Germany). C6 cell line was purchased from Cell Bank of Pasteur Institute (Iran) and rats were bought from Royan Institute (Iran).
Preparation of methotrexate-loaded PLGA nanoparticles
Methotrexate-loaded PLGA nanoparticles were prepared via the precipitation method pursuant to our previous study [21]. MTX and PLGA were dissolved in 5 mL of acetone and added dropwise to the 100 ml of PVA 1% W/V. After stirring overnight, the nanoparticles were obtained by centrifugation at 12000 rpm for 30 min.
Characterization of nanoparticles
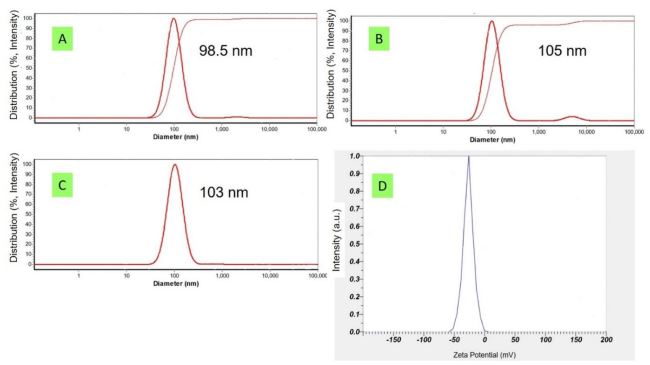
DLS (ScatterScope1) and zeta analyzer (Malvern) were applied for nanoparticles characterization. The percent of drug loading was measured via UV/Vis spectroscopy Cecil (CE 7250, England) at 300 nm.
In vitro cytotoxicity
The cytotoxicity of the nanoparticles was assessed via MTT assay. C6 seeded cells were treated methotrexate-loaded PLGA nanoparticles for 48 and afterwards, washed with PBS and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) solution was inserted and incubated for 3 h. DMSO (Dimethyl sulfoxide) was inserted into each cell followed by shaking and at the end, the absorption was read at 570 nm via microplate reader (Bio Tek 800 TS).
In vivo efficacy
The anti-tumor potential of nanoparticles was studied on C6 GBM in male Wistar rats (220 g). The methotrexate-loaded PLGA nanoparticles were injected via tail vain every week (1 mg/mL) and the tumor size was observed via small animal MRI (Siemens Healthcare, Erlangen, Germany; slew rate, 200 mT/m/ ms; maximum amplitude, 45 mT/m). All steps and protocols were approved by the Animal Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.614).
Results and discussion
The methotrexate-loaded PLGA nanoparticle hydrodynamic mean diameter was 102±5 nm with a zeta potential amount of -26±2 mV (Figure 1).
According to the MTT assay it was observed that 100%, 98%, 91.3%, 82.5%, 69.9% and 56% of cells lived at the concentration of 1, 10, 25, 50, 100 and 200 µg/ml, respectively after 48 h of the incubation with methotrexate-loaded PLGA nanoparticles.
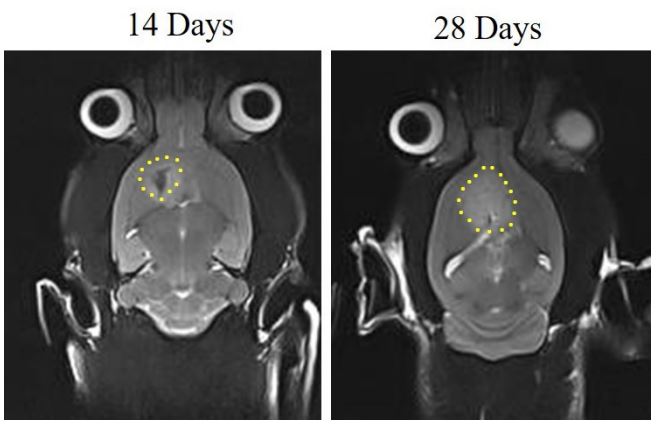
GBM-bearing rats were imaged via MRI 14 and 28 days after tumor implantation and the anti-tumor potential of methotrexate-loaded PLGA nanoparticles was assessed. The tumor size was partially increased (Figure 2) but rats survived for 31 days on average.
Conclusion
In the current study methotrexate-loaded PLGA nanoparticles were prepared and the main approach was to check their antitumor efficacy on GBM-bearing rats. According to the results Methotrexate-loaded PLGA nanoparticles were able to extend both the survival and time for tumor size increment compared to the control. It seems that these nanoparticles can work promisingly in cases that accompany targeted drug delivery and other chemotherapeutics.
Declarations
Acknowledgments: This work was supported by Tehran University of Medical Sciences, Grant No. 9711148003, Iran. The authors would like to acknowledge Preclinical Lab, Core Facility, Tehran University of Medical Sciences, Tehran, Iran and Iranian National Brain Mapping Laboratory (NBML) for providing the in vivo imaging and image processing services for this research
Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carrano A, Juarez JJ, Incontri D, Ibarra A, Cazares HG. Sex-specific differences in glioblastoma. Cells. 2021; 10: 1783.
- Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Advanced drug delivery reviews. 2021; 171: 108-38.
- Huang CW, Chuang CP, Chen YJ, Wang HY, Lin JJ, et al. Integrin α2β1-targeting ferritin nanocarrier traverses the blood–brain barrier for effective glioma chemotherapy. Journal of nanobiotechnology. 2021; 19: 1-17.
- Van Vulpen M, Kal HB, Taphoorn MJ, El Sharouni SY. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? Oncology reports. 2002; 9: 683-8.
- Cao Y, Jin L, Zhang S, Lv Z, Yin N, et al. Blood-brain Barrier Permeable and Multi-stimuli Responsive Nanoplatform for Orthotopic Glioma Inhibition by Synergistic Enhanced Chemo-/Chemodynamic/Photothermal/Starvation Therapy. European Journal of Pharmaceutical Sciences. 2023; 180: 106319.
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. Journal of inherited metabolic disease. 2013; 36: 437-49.
- Caprifico AE, Foot PJ, Polycarpou E, Calabrese G. Overcoming the blood-brain barrier: Functionalised chitosan nanocarriers. Pharmaceutics. 2020; 12: 1013.
- Dong CY, Huang QX, Cheng H, Zheng DW, Hong S, et al. Neisseria meningitidis opca protein/MnO2 hybrid nanoparticles for overcoming the blood-brain barrier to treat glioblastoma. Advanced Materials. 2022; 34: 2109213.
- Saha S, Yakati V, Shankar G, Jaggarapu MMCS, Moku G, et al. Amphetamine decorated cationic lipid nanoparticles cross the blood-brain barrier: Therapeutic promise for combating glioblastoma. Journal of Materials Chemistry B. 2020; 8: 4318-30.
- Liu L, Chen Q, Wen L, Li C, Qin H, et al. Photoacoustic therapy for precise eradication of glioblastoma with a tumor site bloodbrain barrier permeability upregulating nanoparticle. Advanced Functional Materials. 2019; 29: 1808601.
- Zhang C, Song J, Lou L, Qi X, Zhao L, et al. Doxorubicin‐loaded nanoparticle coated with endothelial cells‐derived exosomes for immunogenic chemotherapy of glioblastoma. Bioengineering & Translational Medicine. 2021; 6: e10203.
- Zou Y, Wang Y, Xu S, Liu Y, Yin J, et al. Brain co‐delivery of temozolomide and cisplatin for combinatorial glioblastoma chemotherapy. Advanced Materials. 2022; 34: 2203958.
- Madani F, Esnaashari SS, Webster TJ, Khosravani M, Adabi M. Polymeric nanoparticles for drug delivery in glioblastoma: State of the art and future perspectives. Journal of Controlled Release. 2022; 349: 649-61.
- Mujokoro B, Madani F, Esnaashari SS, Khosravani M, Adabi M. Combination and co-delivery of methotrexate and curcumin: Preparation and in vitro cytotoxic investigation on glioma cells. Journal of Pharmaceutical Innovation. 2020; 15: 617-26.
- Maksimenko O, Malinovskaya J, Shipulo E, Osipova N, Razzhivina V, et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. International journal of pharmaceutics. 2019; 572: 118733.
- Wang S, Yu Y, Wang A, Duan X, Sun Y, Wang L, et al. Temozolomide hexadecyl ester targeted plga nanoparticles for drug-resistant glioblastoma therapy via intranasal administration. Frontiers in Pharmacology. 2022; 13: 965789.
- Bedoui Y, Guillot X, Sélambarom J, Guiraud P, Giry C, et al. Methotrexate an old drug with new tricks. International journal of molecular sciences. 2019; 20: 5023.
- Hannoodee M, Mittal M. Methotrexate. StatPearls [Internet]: StatPearls Publishing. 2022.
- Lopes DV, de Fraga Dias A, Silva LFL, Scholl JN, Sevigny J, et al. Influence of NSAIDs and methotrexate on CD73 expression and glioma cell growth. Purinergic Signalling. 2021; 17: 273-84.
- Capelôa T, Caramelo F, Fontes-Ribeiro C, Gomes C, Silva AP. Role of methamphetamine on glioblastoma cytotoxicity induced by doxorubicin and methotrexate. Neurotoxicity research. 2014; 26: 216-27.
- Madani F, Esnaashari SS, Bergonzi MC, Webster TJ, Younes HM, et al. Paclitaxel/methotrexate co-loaded PLGA nanoparticles in glioblastoma treatment: Formulation development and in vitro antitumor activity evaluation. Life sciences. 2020; 256: 117943