SciBase Journals
SciBase Oncology
ISSN 2996-3826
- Article Type: Review Article
- Volume 2, Issue 1
- Received: Mar 12, 2024
- Accepted: Jun 27, 2024
- Published Online: Jul 04, 2024
Research Progress on the Mechanism of Chinese Herbal Compounds in the Treatment of Benign Prostatic Hyperplasia
Dongyue Ma1†; Haoling Liu2†; Zihao Li3 ; Shengyao Li4 ; Dexiu Li5 ; Guanchao Du1*
1Department of Andrology, China Academy of Chinese Medical Sciences, Xiyuan Hospital, Beijing, China.
2School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China.
3Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China.
4Department of Neuropathy, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China.
5National Clinical Research Center for Chinese Medicine Cardiology, China Academy of Chinese Medical Sciences, Xiyuan Hospital, Beijing, China.
†Equal Contributors.
*Corresponding Author: Guanchao Du
National Clinical Research Center for Chinese Medicine
Cardiology, Xiyuan Hospital of China Academy of Chinese
Medical Sciences, No. 1 Xiyuan Caochang, Zhongzhi Road,
Haidian District, Beijing 100091, China.
Tel: +86-18001392186;
Email: duguanchao@163.com
Abstract
Benign Prostatic Hyperplasia (BPH) is one of the most common benign diseases in middle-aged and elderly men, characterized by lower urinary tract symptoms such as urinary frequency, urgency, and weak urine flow, which can lead to complications such as urinary retention and incontinence. Traditional Chinese Medicine (TCM) has a long history and rich experience in treating BPH, with herbal formulas widely used in clinical practice due to their significant efficacy and safety. This study aims to explore the therapeutic effects and potential mechanisms of Chinese herbal compounds on BPH through a comprehensive analysis of previous research, systematically summarizing the mechanisms of Chinese herbal compounds in treating BPH to provide a theoretical basis for the development of new TCM drugs. The research findings reveal that the specific mechanisms of Chinese herbal compounds in treating BPH involve the modulation of sex hormones, prostate proliferation and apoptosis, cell growth factors, inflammation and oxidative stress, as well as epithelial-mesenchymal transition.
Keywords: Benign prostatic hyperplasia; Chinese herbal compounds; Animal model; Mechanism; Sex hormones.
Citation: Ma D, Liu H, Li Z, Li S, Li D, Du G. Research Progress on the Mechanism of Chinese Herbal Compounds in the Treatment of Benign Prostatic Hyperplasia. SciBase Oncol. 2024; 2(1): 1013.
Introduction
Benign Prostatic Hyperplasia (BPH) is a common urological disease affecting middle-aged and elderly men. With the exacerbation of global population aging, the incidence of BPH is rapidly increasing. Over 50% of men over 50 years old have an enlarged prostate volume, and in men over 80 years old, this proportion reaches 90% [1]. Currently, surgical and pharmacological treatments are the two main therapeutic strategies for BPH in clinical practice [2]. Surgery may subject patients to psychological stress, especially for those with large prostate enlargement, with postoperative complications such as hematuria, urinary difficulties, and a higher risk of urinary tract infections [3]. Additionally, elevated prostate-specific antigen levels may indicate a risk of recurrence or progression to prostate cancer [4]. Given the demographic changes and the severity of prostate enlargement worldwide, targeted prevention and control of BPH are imperative
The main theories of BPH pathogenesis include DHT, stromal-epithelial interactions, androgen-estrogen coordination, embryonic reawakening, and disturbances in cell proliferation and apoptosis, although the specific inducement mechanisms remain unclear [5]. Only 5-alpha reductase inhibitors significantly reduce prostate volume in BPH treatment, but currently used drugs such as dutasteride and finasteride have significant side effects [6]. These issues underscore the urgency of developing multi-target drugs with minimal side effects and toxicity. TCM has been used clinically to treat various types of diseases, including BPH. TCM treatment for BPH has shown good efficacy, significantly improving a range of lower urinary tract symptoms with relatively few side effects [7]. The pharmacological basis of TCM for treating prostate enlargement has been a hot topic of research worldwide, and Chinese herbal compounds are considered a promising treatment method for BPH due to their multi-component, multi-target nature. Animal experiments targeting BPH have shown that TCM intervention can significantly reduce prostate weight, prostate index, and pathological changes in prostate tissue in model rats [8]. To further elucidate the mechanism of action of Chinese herbal compounds in treating BPH, based on the pharmacological research of Chinese herbal compounds for BPH treatment, we review their specific mechanisms of action to provide insights into the mechanism of TCM treatment for BPH.
Effect on sex hormones
Androgens and the androgen receptor signaling pathway play a crucial role in the occurrence and development of BPH [9]. During the development of BPH, 5-Alpha Reductase (5-AR) catalyzes the conversion of testosterone to Dihydrotestosterone (DHT), leading to an increase in DHT levels in the prostate. DHT has a higher affinity for the androgen receptor and can stimulate protein synthesis, differentiation, and prostate cell growth [10]. Most of the estrogen is synthesized from androgens produced by the adrenal glands and testes through aromatase action. Estrogen can induce the expression of Transforming Growth Factor Beta 1 (TGF-β1) in prostate stromal cells through non-genomic activation of the mitogen-activated protein kinase pathway, enhance the phenotype of stromal smooth muscle cells, and promote proliferation of prostate epithelial cells [11]. Furthermore, abnormal expression of aromatase in BPH and malignant prostate tissues increases the ratio of E2 to T, but maintaining the balance between E2 and T is crucial for prostate health [12]. Long Dan Xie Gan Tang, Paljung-san, Zishen Pill, and Bawu Decoction can inhibit the development of BPH by suppressing serum DHT, indicating their potential role in inhibiting 5-AR [13-15]. Animals treated with Xialiqi and Liu Wei Di Huang Tang showed a significant decrease in absolute and relative prostate weights, as well as reduced levels of DHT in serum and prostate, and mild prostate epithelial hyperplasia accompanied by decreased expression of Proliferating Cell Nuclear Antigen (PCNA) [8,16]. Bushen Tongluo formula and Kangquan Recipe can regulate and improve the ratio of estrogen to androgen in BPH rats, which is one of their main mechanisms for treating BPH [5,17].
Effect on vascular growth factor
Angiogenesis plays a crucial role in the progression and development of BPH and has become an attractive target for BPH treatment. Angiogenesis is a complex process regulated by various molecules, among which growth factors are one of the important regulators of angiogenesis. They can promote the proliferation and migration of endothelial cells, thereby facilitating the formation of new blood vessels, and consequently accelerating the development of BPH. Therefore, targeting growth factors and their regulated angiogenesis pathways may be an effective strategy for treating BPH. Nerve Growth Factor (NGF) plays a key role in developmental neurobiology due to its important neuronal functions. Neuronal hypertrophy was observed after partial urethral obstruction in rats, and increased expression of NGF was reported to be a major factor. Basic fibroblast Growth Factor (bFGF) exists in prostate tissue and can promote fibroblast mitosis. The expression of bFGF in BPH tissue is much higher than in normal prostate tissue, and it increases with the growth of BPH stromal components, suggesting that bFGF may play a role in the occurrence and development of BPH [18]. TGF-β1 may have a dual role in BPH. Some scholars believe that transforming growth factor TGF-β1 and its receptor induce the aggregation and proliferation of prostatic stromal cells by stimulating fibroblast growth factor, epidermal growth factor, and other combined reactions, leading to the occurrence of BPH [19]. Zi-Shen Pill can promote apoptosis of prostate cells by increasing TGF-β1 levels to achieve therapeutic purposes [20]. The occurrence of BPH also involves the microvascular system. Vascular Endothelial Growth Factor (VEGF) is responsible for angiogenesis in the gland and is released by epithelial cells under the action of androgens. It is a pro-angiogenic factor mediated by the PI3K/Akt signaling pathway, promoting angiogenesis and cell proliferation in the stromal tissue, causing proliferative growth and inducing BPH [21]. Bushen Tongluo Formula can effectively inhibit the proliferation of prostate tissue by improving the imbalance of serum hormone levels and the expression of vascular endothelial growth factor in rat models, thereby promoting the local blood circulation of the prostate [17]. Qianlie Tongqiao Capsule may alleviate BPH symptoms by targeting the expression of NGF, bFGF, and TGF-β1 in the bladder, which are involved in bladder dysfunction and remodeling associated with BPH [22].
Effect on apoptosis and cell proliferation
The occurrence and development of BPH are attributed to the decreased apoptosis rate of prostate cells and excessive proliferation of stromal cells, which are physiological responses to maintaining their cell number and functional activities [23]. The proliferation and apoptosis of prostate cells are in dynamic balance, and the imbalance between proliferation and apoptosis of prostate cells is closely associated with BPH [24]. The process of apoptosis is regulated by the B-cell lymphoma 2 (Bcl-2) protein family, which mainly consists of anti-apoptotic proteins such as Bcl-2 and B-cell lymphoma-extra-large (Bcl-XL), and proapoptotic proteins such as Bcl-2-associated X protein (Bax) [25]. The apoptosis signal ultimately requires the activation of Caspase enzymes to execute. Among them, caspase enzymes, particularly caspase-9 as a crucial initiator, play important roles and can activate downstream effector molecules such as caspase-3 to induce apoptosis cascade reactions [26]. PCNA and Ki67 are mainly synthesized intracellularly and are associated with the cell cycle, peaking during the G1-S phase, used to evaluate the degree of cell proliferation activity [27]. Generally, p15, p18, p21, and p27 are considered anti-proliferative molecules, while cyclin B, cyclin D, cyclin E, as well as cdk2 and cdk4, are considered pro-proliferative molecules [28].
Bushen Tongluo formula significantly reduces the expression levels of TGF-β1 and Ki67 in the prostate tissue of BPH rats while increasing the expression of caspase-3. Moreover, in cell experiments, it inhibits the proliferation and motility of BPH-1 cells by blocking the cell cycle progression in the S or G2M phase and promotes early or late apoptosis of BPH-1 cells [17]. Long Dan Xie Gan Tang and Paljung-San can lower the expression levels of Ki-67 and cyclin D1 in the prostate of BPH rats, inhibiting prostate cell proliferation by arresting the cell cycle in the S phase [13,29]. Danzhi Qing’e Tang reduces Ki-67, cyclin D1, and PCNA protein levels, decreases DNA synthesis, and arrests the cell cycle in the S phase to inhibit the proliferation of BPH-1 cells [30]. Qianlongtong strongly induces apoptosis of prostate cells, increases the G0/G1 phase of benign hyperplastic prostate cells, and decreases the G/M phase, regulating the treatment mechanism of BPH, possibly by downregulating the antiapoptotic molecule Bcl-2 and upregulating the pro-apoptotic molecule Bax [31]. Additionally, Qianliening Capsules inhibit the proliferation of BPH-1 cells by regulating miRNA expression, involving 107 upregulated genes and 71 downregulated genes, mainly affecting key pathways such as cell proliferation and apoptosis [32].
Effects on inflammation and oxidative stress
The relationship between inflammation and oxidative stress forms a vicious cycle, mutually promoting and exacerbating each other’s occurrence and development. Controlling inflammatory responses and oxidative stress is crucial for preventing and treating BPH. Inflammatory infiltration in prostate tissue suggests that chronic inflammation may be one of the pathogenic mechanisms of BPH. Previous studies have found that Chinese herbal medicine cinnamon can prevent oxidative stress reactions by reducing the levels of lipid peroxidation product Malondialdehyde (MDA) and increasing the levels of the antioxidant Glutathione (GSH), thereby lowering the levels of pro-inflammatory factors TNF-α and IL-1β in the serum, thus improving arthritis [33]. The occurrence and development of inflammatory diseases are closely related to the metabolism of Arachidonic Acid (AA) [34]. The metabolism of AA includes pathways such as Cyclooxygenase (COX), Lipoxygenase (LOX), and cytochrome P450 [35]. COX-2 and 5-LOX are considered key enzymes in AA metabolism [36]. The occurrence of BPH may be accompanied by overexpression of COX-2 and 5-LOX [13]. Therefore, exploring inhibitors of inflammation and oxidative stress from traditional prescriptions may also become a new strategy for treating BPH. Paljung-San, a traditional herbal formula, regulates the inflammatory response in BPH-1 cells by inhibiting the production of prostaglandin E2 and reducing the protein level of COX-2, and restores the activity of glutathione reductase and the level of malondialdehyde in the prostate tissue of BPH rats, exhibiting antioxidant activity [13]. Long Dan Xie Gan Tang inhibits oxidative stress in the prostate of BPH rats, as evidenced by the reduction in lipid peroxidation marker Malondialdehyde (MDA) concentration and increase in glutathione reductase activity, thereby preventing the occurrence of BPH [37]. The mechanism of action of Xialiqi in treating BPH involves both anti-inflammatory and antioxidant stress aspects, significantly reducing MDA, Tumor necrosis factor alpha (TNF-α), and Interleukin-8 (IL-8) levels in BPH rats, increasing Superoxide Dismutase (SOD) activity, and thus protecting prostate cell structure [16]. Kelong Capsules likely exert their therapeutic effects in treating BPH by increasing the expressions of Nuclear factor erythroid 2-related factor 2 (Nrf-2) and NAD(P)H quinone dehydrogenase 1 (NQO1), which are involved in antioxidant defense mechanisms, and by upregulating COX-2 protein expression, potentially modulating the inflammatory response associated with BPH [38]. Buzhong Yiqi decoction may alleviate symptoms of BPH by decreasing the levels of pro-inflammatory cytokines, thereby reducing inflammation in the prostate tissue [39].
Impact on Epithelial-Mesenchymal Transition (EMT)
EMT, referring to the biological process in which epithelial cells lose polarity and connections with the basement membrane and other epithelial cell phenotypes, transforming into mesenchymal phenotypes with high migration and invasion capabilities, as well as resistance to apoptosis. EMT is typically a biological process of tumor cell formation, migration, and invasion, but it is also closely related to the pathogenesis and progression of BPH. In BPH, some epithelial cells lose expression of E-cadherin while gaining expression of vimentin, indicating the presence of EMT phenomenon [40]. During the EMT process, E-cadherin is gradually replaced by N-cadherin, vimentin, and other interstitial cell molecules [41]. The TGF-β/Smad pathway plays a crucial role in EMT in prostate tissue, where TGF-β forms a complex with Smad, enters the nucleus, and binds to Smad binding elements in the promoter region of EMT-related genes, thereby promoting EMT in prostate tissue. In the testosterone propionate-induced BPH model, Kangquan Recipe can inhibit the transmission of the TGF-β/Smad signaling pathway by upregulating the expression of BAMBI protein in rat prostate tissue, thereby reversing EMT [42]. Bushen Tongluo Formula may alleviate BPH symptoms by reducing fibrosis and EMT in the prostate tissue, as evidenced by decreased levels of TGF-β1 and vimentin, and by promoting epithelial integrity through the upregulation of E-cadherin expression [17].
Table 1: Summary of the mechanism treatment BPH used CHMs (Herbal Formulas).
| Formulas | Ingredients | Action pathway/mediator site | Mechanism | Animal Model | Result |
|---|---|---|---|---|---|
| Xia Li Qi [16] | Huang Qi (Astragali Radix), Nv Zhen Zi (Ligustri Lucidi Fructus), Rou Gui (Cinnamoni Cortex), Hua shi (Talcum), Huang Bai (Phellodendri Amurensis Cortex), Hu Po (Amber), Xia Ku Cao (Prunellae Spica), Li Zhi He (Litchi Semen). |
↓Levels of IL-8, TNF-α, DHT, MDA, and PCNA in the prostate. ↑Activity of SOD in the prostate. ↑Levels of caspase-3 in the prostate. |
Regulation of sex hor- mones; Pro-apoptot- ic; Anti-inflammatory; Anti-oxidative. |
Castrated rats received subcutaneous injections of TP at 0.5 mg/kg/d. |
↓PW, PV, and PI. |
| Kangquan Recipe [5,42,43] |
Ba Ji Tian(Radix morinda officinalis), Tu Bie Chong(eupolyphaga seu steleophaga), Di Long(pheretima), Da Huang(Radix et Rhizoma Rhei), Gui Zhi(Ramulus Cimmamomi), Luo Shi Teng (caulis trachelospermi) |
↓ Levels of plasma testosterone in BPH rats. ↑ Levels of plasma E2 in BPH rats. ↑Expression of BAMBI and E-cadherin in the hypothalamus, pituitary and prostate tissue. ↓Expression of PCNA, TGF-β, TGF-βR1, TGF-βR2, p-Smad2, p-Smad3, and N- cadherin protein in prostate tissue. |
Regulation of sex hormones; Reversing EMT. |
Castrated rats received subcutaneous injections of TP for 30 days. |
↓PW, PV, PI and Pros- tatic epithelial tissue proliferation. |
| Bawu Decoction [15] |
Di Huang(Rehmannia glutinous), Fu Ling(Sclerotium of Wolfi poria cocos), Dang Gui(Angelica gigas), Bai Shao(Paeonia lactiflora), Ren Shen(Panax ginseng), Bai Zhu(Atractylodes japonica), Chuan Xiong(cuidium officinale) Gan Cao(Glycyrrhiza uralensis) |
↓ Levels of serum testosterone and DHT in BPH rats. |
Regulation of sex hormones |
Castrated rats received subcutaneous injections of TP at 10 mg/kg (dis- solved in corn oil). |
↓PW and thickness of epithelium tissue from prostate |
| Qianliening Capsules [32,44-49] |
Da Huang (rhubarb), Shui Zhi(leech), Huang Qi(astragalus), Niu Xi(achyranthes), Tu Si Zi(dodder) |
↑Expression of Bax/Bcl-2 ratio in BPH rats. ↓mRNA and protein expression of collagen IV, fibronectin, Bcl-2 and cyclin D1 of BPH-1 cells ↓Expression of Bcl-2, cyclin D1 and CDK4 in WPMY-1 cells ↑Expression of Bax and p21 in WPMY-1 cells ↓Activation of the STAT3 signaling pathway in WPMY-1 cells. ↑Activation of Caspases-3,9 in BPH rats. ↓Expression of HIF-1α, VEGF and bFGF in BPH rats. ↓Expression of PCNA, cyclin D1 and CDK4 in prostatic tissue of BPH rats. ↓Proliferation ability and G1/S progression of bFGF-stimulated WPMY-1 cells. |
Regulation of growth factors; Pro-apop- totic; Antiangiogenic activ- ity in prostatic tissue of BPH rats. Regulation of miRNA. |
Castrated rats received subcutaneous injections of TP at 5 mg/kg. WPMY-1 cells and BPH- 1 cells; |
↓Morphological integrity, number and viability of the BPH-1 cells. |
| Kelong Capsules [38] |
Dihuang(Rehmanniae Radix Praeparata), Shanzhuyu(Cornus Fructus), Fuling(Poria), Mu Dan Pi(Moutan Cortex), Gui zhi (Cinnamomi Ramulus); Fu Zi(Aconm Lateralis Radix Praeparaia); Huangq Qi (Astragali Radix); Ci Shi (Magnetitum). |
↑Expressions of Nrf-2 and NQO1 in BPH rats. ↑Expression of COX-2 proteinin BPH rats |
Anti-inflammatory; Anti-oxidative. |
Castrated rats received subcutaneous injec- tions of 5 mg/kg TP for 28days. |
↓PI, pathological changes in the pros- tate and kidney. |
| Qianliexin Capsule [50,51] |
Dan Shen (Salvia miltiorrhiza Bunge), Mo Yao (Commiphora myrrha) , Taoren (Prunus persica (L.) Batsch), Chichao (Paeonia lactiflora Pall.), Honghua (Carthamus tinctorius L.), Zelan (Lycopus lucidus Turcz. ex Benth.), Wangbuliuxing (Gypsophila vaccaria (L.) Sm.), Zaojiaoci(Gleditsia sinensis Lam.) Baijiangcao (Patrinia scabiosifolia Link), Pugongying(Taraxacum mongolicum Hand.-Mazz.), Chuanlianzi(Melia azedarach L.), Baizhi(Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav.), Shiwei (Pyrrosia sheareri (Baker) Ching), Gouqizi (Lycium barbarum L.) |
↓Level of NF-κB, p62, NLRP3, ASC, IL-1β and TNF-α in prostate tissue. ↓Level of MDA in prostate tissue. ↑Activities of SOD, CAT, and GSH in pros- tate tissue. ↓Expression of TGF-β, EGFR, and collagen in BPH cells. ↓Levels of IL-1β and TNF-α in prostate tissue. ↓Levels of urea nitrogen, creatinine, and uric acid. |
Pro-apoptotic; Anti-inflammatory; Anti-oxidative; Up-regulation of au- tophagy in both CNP and BPH rat models; Effect on Fibrosis- related Markers; Effect on Renal Func- tion and Metabolism |
Male rats undergo sham castration followed by 17β-estradiol (0.25 mg/kg) injection for 20 days; castration fol- lowed by TP (5 mg/kg) injection for 33 days, both starting on day 3 post-surgery; Male rats are surgically castrated and injected with 1% carrageenan (CGN) into the prostate vein three days later. |
↓PI, epithelial thickness, LUTS (pain response time, urine volume, prostatic blood flow, clotting time) in CGN rat models; ↓ infiltration of inflammatory cells (Ly6G+ cells) in prostate tissue from CGN rat models. ↑urine volume and renal function indica- tors. ↓Fibrotic damage. |
| Zi-Shen Pill [14,20, 52,53] |
Zhi Mu (Anemarrhenae Rhizoma), Huang Bai(Phellodendri Chinensis Cortex), Rou Gui (Cinnamomi Cortex). |
Regulating AA metabolism through refrain the expression of COX and LOX. ↓Levels of DHT and testosterone in serum. ↓Expression of VEGF, bFGF in the rat prostates. ↑Expression of TGF-β1 in the rat prostates. |
Regulation of sex hormones; Regulation of growth factors; Anti-inflammatory. |
Castrated rats were given 0.5 mg/kg of TP subcutaneously in olive oil along with 10 mL/kg of saline for 4 weeks. |
↓PW, PI and patho- logical changes in BPH rats. |
| Paljung- san [13] |
Da Huang (Rhei Radix et Rhizoma), Mu Tong (Akebiae Caulis), Qu Mai(Dianthi Herba), Bian Xu(Polygoni Avicularis Herba), Hua Shi (Talcum); Zhi Zi (Gardeniae Fructus), Che Qian Zi (Plantaginis Semen), Gan Cao (Glyc- yrrhizae Radix et Rhizoma), Deng Xin Cao(Junci Medulla). |
↓Expression of proliferation markers such as Ki-67, cyclin D1 and PCNA protein levels in vivo and vitro. ↓Levels of PGE2 and COX-2 protein in BPH-1 cells. ↓Levels of DHT in the prostates ↑GR activity in the prostates. ↓Levels of MDA in the prostates. |
Regulation of sex hormones; Anti-proliferative; Anti-inflammatory; Anti-oxidative. |
Castrated rats were given 3mg/kg of TP subcutaneously in olive oil for 4 weeks; BPH-1 cells. |
↓PW, BPH-1 cell viability; ↑Prostatic morphol- ogy. |
| Long Dan Xie Gan Tang [29,37] |
Long Dan Cao (Gentiana Scabra Radix), Chai Hu (Bupleuri Radix), Ze Xie (Alismatis Rhizoma), Mu Tong (Akebia Caulis), Che Qian Zi (Plantaginis Se- men), Fu Ling (Poria Sclerotium), Sheng Di Huang (Rehmanniae Radix Crudus), Bai Zhi (Angeli- cae Gigantis Radix), Zhi Zi (Gardeniae Fructus), Huang Qin (Scutellariae Radix), Gan Cao (Glycyr- rhizae Radix et Rhizoma). |
↓Concentrations of testosterone and DHT in the prostate; ↓Expression of PCNA, cyclin D1, and Ki-67 in vivo and vitro. ↓Levels of PGE2, COX-2, IL-8 and IL-6 in BPH- 1 cells. ↓MDA concentration in the prostate. ↑GR activity in the prostate. |
Regulation of sex hor- mones; Anti-prolifer- ative; Anti-inflammatory; Anti-oxidative. |
Castrated rats were giv- en 3mg/kg of TP subcu- taneously in olive oil for 4 weeks; BPH-1 |
↓relative prostate weight and alleviates histological abnor- malities associated with BPH |
| Liu Wei Di Huang Tang [8] |
Shu Di Huang (Rehmanniae Radix Praeparata), Shan Zhu Yu (Corni Fructus), Shan Yao (Di- oscoreae Rhizoma), Mu Dan Pi (Moutan Cortex), Fu Ling (Poria), Ze Xie (Alismatis Rhizoma) |
↓Levels of DHT in Serum and prostatic. ↓Expression of PCNA protein in the prostate. |
Regulation of sex hormones; Anti-proliferative. |
Castrated rats were given 3mg/kg of TP subcutaneously in olive oil for 4 weeks. |
↓PW and Prostatic epithelial hyperplasia. |
| Bushen Tongluo Formula [17] |
Tu Si Zi(Cuscuta chinensis Lam.), Huang Bai(Phellodendron amurense Rupr.), Di Long(Pheretima aspergillum (E. Perrier), Tu Bie Chong(Eupolyphaga seu Steleophaga) |
↓Serum levels of E2 and DHT in BPH rats. ↓Levels of TGF-β1 and vimentin in prostate cells. ↑Epithelial markers E-cadherin in prostate cells. ↓BPH-1 cell activity. ↑Early apoptotic cell. ↑G0/G1 phase in BPH-1 cells. ↓S phase in BPH-1 cells. |
Regulation of sex hormones; Anti-proliferative; Pro-apoptotic; Inhibite of EMT. |
Castrated rats were given 3mg/kg of TP subcutaneously in olive oil for 4 weeks. BPH-1 cells |
↓PV, PI and histo- pathological changes. |
| Buzhong Yiqi Decoction [39] |
Huangqi (Radix Astragali Mongolici), Baizhu (Rhizoma Atractylodis Macrocephalae), Chenpi (Pericarpium Citri Reticulatae), Shengma (Rhizoma Cimicifugae Foetidae), Chaihu (Radix Bupleuri Chinensis), Renshen (Radix Ginseng), Gancao (Radix Glycyrrhizae), Danggui (Radix Angelicae Sinensis) |
↓Levels of IL-6, TNF-α and IL-1β in vitro. |
Anti-inflammatory | BPH-1 cells. | ↓Proliferating activity of BPH- 1 cells. |
| Dan zhi qing’e Decoction [30] |
Huangqi (Radix Astragali Mongolici), Baizhu (Rhizoma Atractylodis Macrocephalae), Chenpi (Pericarpium Citri Reticulatae), Shengma (Rhi- zoma Cimicifugae Foetidae), Chaihu (Radix Bupleuri Chinensis), Renshen (Radix Ginseng), Gancao (Radix Glycyrrhizae), Danggui (Radix Angelicae Sinensis) |
↓Levels of CD68-positive cells in prostate tissue. ↑ Levels of CD206-positive cells in prostate stroma. ↓Levels of TNF-α, IL-1β, MCP-1, TGF-β, and IL-17 mRNA expression in prostate tissue. ↓Serum levels of TNF-α and IgG. ↓ PCNA-positive cells in prostate epithe- lium. ↓ Phosphorylation of ERK1/2 in vivo and vitro. |
Anti-inflammatory; Anti-proliferative. |
Experimental autoim- mune prostatitis (EAP)- induced BPH; M2 macrophage-stimu- lated BPH-1 cells. |
↓ Prostate enlarge- ment, PI value in EAP rats. |
| Qianlie Tongqiao Capsule [22] |
Huai niu xi (Radix achyranthis bidentatae), Yi zhi ren (Semen amomi amari), Wu yao (Radix lin- derae), Rou gui (Cinnamomum cassia), Shui zhi (Aulastomum gulo), Tu si zi (Semen cuscutae), Huang qi (Astragalus mongholicus). |
↓Expression of NGF, bFGF and TGF-β1 in the bladder of BPH rats. ↓Expression of Bcl-2/Bax ratio in the blad- der of BPH rats. |
Regulation of growth factors; Anti-proliferative; Pro-apoptotic. |
Castrated rats were given 5mg/kg of TP subcutaneously in olive oil for 4 weeks. |
↓ Bladder weight and bladder index in BPH rats. ↑ Structural and functional changes in the obstructed bladder. ↓ Detrusor thickness in BPH rats. |
| Qianlon gtong [31] |
Huang qi (Astragalus membranaceus), Pu haung (Scutellaria baicalensis), San qi (Panax notoginseng), Yi Mu Cao (Leonurus heterophyllus), Wang bu liu xing (Eclipta prostrata), San leng (Trillium tschonoskii), E zhu(Curcuma phaeocaulis), Pao shan jia(Lithospermum erythrorhizon), et al. |
↑G0/G1 phase in hyperplastic prostate cells. ↓G/M phase in hyperplastic prostate cells. ↑mRNA expression of caspase3, Bax/Bcl-2 ratio in hyperplastic prostate cells. |
Anti-proliferative; Pro-apoptotic. |
Benign hyperplastic prostate cells |
Inhibits proliferation and promotes the apoptosis of hyper- plastic prostate cells. |
Abbreviations: ↓: inhibited or alleviated; ↑: upregulated or increased; IL-8: Interleukin-8; TNF-α: Tumor Necrosis Factor-alpha; DHT: Dihydrotestosterone; MDA: Malondialdehyde; PCNA: Proliferating Cell Nuclear Antigen; SOD: Superoxide Dismutase; BAMBI: BMP and Activin Membrane-Bound Inhibitor; EMT: Epithe- lial-to-Mesenchymal Transition; TGF-β: Transforming Growth Factor-beta; Smad: Small Mothers Against Decapentaplegic; NGF: Nerve Growth Factor; bFGF: Basic Fibroblast Growth Factor; TGF-βR1: Transforming Growth Factor-beta Receptor 1; TGF-βR2: Transforming Growth Factor-beta Receptor 2; p-Smad2: Phosphorylated Smad2; p-Smad3: Phosphorylated Smad3; N-cadherin: Neural Cadherin; STAT3: Signal Transducer and Activator of Transcription 3; HIF-1α: Hypoxia-Inducible Factor 1-alpha; VEGF: Vascular Endothelial Growth Factor; bFGF: Basic Fibroblast Growth Factor; NLRP3: NOD-like Receptor Pyrin Domain Containing 3; ASC: Apoptosis- associated Speck-like protein containing a CARD; NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells; GSH: Glutathione; EGFR: Epidermal Growth Factor Receptor; Ki-67: Marker of Proliferation Ki-67; GR: Glucocorticoid Receptor; LOX: Lipoxygenase; MCP-1: Monocyte Chemoattractant Protein-1; IL-6: Inter- leukin-6; IL-1β: Interleukin-1beta; IL-17: Interleukin-17; IgG: Immunoglobulin G; CD68: Cluster of Differentiation 68; CD206: Cluster of Differentiation 206; ERK1/2: Extracellular Signal-Regulated Kinase 1/2; EMT: Epithelial-to-Mesenchymal Transition; TP: Testosterone Propionate; CGN: Carrageenan; EAP: Experimental Autoim- mune Prostatitis; PW: Prostate Weight; PV: Prostate Volume; PI: Prostate Index.
Discussion
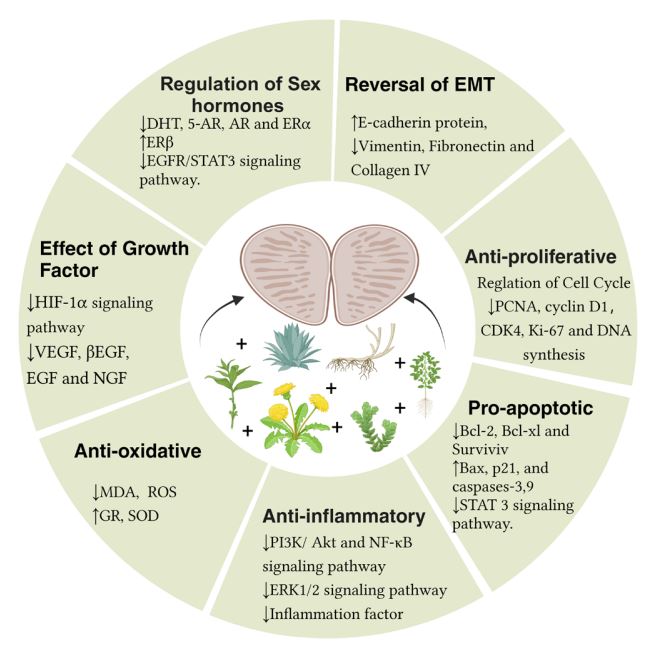
BPH is a progressive disease that clinically manifests with symptoms such as urinary incontinence and urinary retention, greatly affecting patients’ quality of life [54]. The pathological manifestation of BPH involves the proliferation of epithelial and stromal cells around the urethra, forming multiple small nodules that fuse into larger adenomas separated from each other. Enlarged glands, bladder neck obstruction, and increased prostatic smooth muscle tension lead to a series of clinical symptoms, specifically categorized into Benign Prostate Enlargement (BPE), Bladder Outlet Obstruction (BOO)and Lower Urinary Tract Symptoms (LUTS) which overlap and occur inconsistently in sequence, resulting in the complexity of BPH clinical presentations [55]. Many patients seek medical attention only when complications of BPH occur, highlighting the importance of early diagnosis and appropriate treatment. Considering that patients with prostate enlargement are often elderly and frail with multiple comorbidities, TCM has distinct advantages in treating prostate enlargement, not only due to its precise efficacy but also its minimal adverse reactions. Therefore, discovering safe and effective Chinese herbal compounds holds great application value and development potential for better prevention and treatment of prostate enlargement, as emphasized and analyzed in recent years’ research on Chinese herbal compounds and medicinal materials with potential for treating prostate enlargement. The results demonstrate that Chinese herbal compounds can target BPH through multiple mechanisms, as depicted in Figure 1, Table 1.
Despite the numerous drugs available clinically for treating Benign Prostatic Hyperplasia (BPH), they often exhibit shortcomings such as poor anti-inflammatory effects and high incidence of adverse reactions. Chinese herbal compounds, on the other hand, suppress prostatic hyperplasia and alleviate prostatitis-induced inflammatory responses through various pathways, including anti-inflammatory actions, promotion of cell apoptosis, inhibition of epithelial-mesenchymal transition, and suppression of growth factor secretion. This suggests that Chinese herbal compounds may serve as complementary therapies for the clinical treatment of benign prostatic hyperplasia. Based on this, it is hoped that researchers will expedite the development of drugs primarily composed of Chinese herbal compounds, leveraging existing pharmaceutical processing technologies to facilitate larger-scale multicenter clinical studies and elucidate the mechanisms of action of Chinese herbal compounds in preventing and treating benign prostatic hyperplasia. This study underscores the significance of using Chinese herbal compounds to alleviate symptoms of prostate enlargement, laying the groundwork for the development of new TCM drugs for treating prostate enlargement and providing a reference direction for exploring the material basis and mechanisms of action of TCM in treating prostate enlargement.
Declarations
Financial support and funding: This research was funded by the China Academy of Chinese Medical Sciences (ZZ16-XRZ-014) and the Youth Realism Project of the China Association of Chinese Medicine (2023-QNQS-02).
Conflicts of interest: Not applicable.
Sources of support for the work: Not applicable.
Acknowledgments: Not applicable.
Author’s contribution: Guanchao Du: Conceptualization, Methodology and Article framework construction. Dongyue Ma: Writing-Original draft preparation. Haoling Liu: WritingOriginal draft preparation. Zihao Li: Writing-Reviewing and Editing. Shengyao Li: Writing-Reviewing and Editing. Dexiu Li: Legend modification.
Ethics approval and consent to participate: Not applicable.
References
- Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int. 2021; 127: 389-399.
- Gandhi J, Weissbart SJ, Smith NL, et al. The impact and management of sexual dysfunction secondary to pharmacological therapy of benign prostatic hyperplasia. Transl Androl Urol. 2017; 6: 295-304.
- Ottaiano N, Shelton T, Sanekommu G, et al. Surgical Complications in the Management of Benign Prostatic Hyperplasia Treatment. Curr Urol Rep. 2022; 23: 83-92.
- Liu FC, Hua KC, Lin JR, et al. Prostate resected weight and postoperative prostate cancer incidence after transurethral resection of the prostate: A population-based study. Medicine (Baltimore). 2019; 98: e13897.
- Huang YP, Du J, Hong ZF, et al. Effects of Kangquan Recipe on sex steroids and cell proliferation in rats with benign prostatic hyperplasia. Chin J Integr Med. 2009; 15: 289-292.
- Al Saffar H, Xu J, O’Brien JS, et al. US Food and Drug Administration Warning Regarding Finasteride and Suicidal Ideation: What Should Urologists Know? Eur Urol Open Sci. 2023; 52: 4-6.
- Song CS, Guo J, Chang DG, et al. Effect of Longbishu capsule () plus doxazosin on benign prostatic hyperplasia: a randomized controlled trial. Chin J Integr Med. 2014; 20: 818-822.
- Shin IS, Lee MY, Ha HK, et al. Inhibitory effect of Yukmijihwangtang, a traditional herbal formula against testosterone-induced benign prostatic hyperplasia in rats. BMC Complement Altern Med. 2012; 12: 48.
- Tong Y, Zhou RY. Review of the Roles and Interaction of Androgen and Inflammation in Benign Prostatic Hyperplasia. Mediators Inflamm. 2020; 2020: 7958316.
- Madersbacher S, Sampson N, Culig Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A MiniReview. Gerontology. 2019; 65: 458-464.
- Yang Y, Sheng J, Hu S, et al. Estrogen and G protein-coupled estrogen receptor accelerate the progression of benign prostatic hyperplasia by inducing prostatic fibrosis. Cell Death Dis. 2022; 13: 533.
- Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011; 8: 29-41.
- Park E, Lee MY, Jeon WY, et al. Paljung-San, a traditional herbal medicine, attenuates benign prostatic hyperplasia in vitro and in vivo. J Ethnopharmacol. 2018; 218: 109-115.
- Bian Q, Wang W, Wang N, et al. Arachidonic acid metabolomic study of BPH in rats and the interventional effects of Zishen pill, a traditional Chinese medicine. J Pharm Biomed Anal. 2016; 128: 149-157.
- Eom JH, Cheon SY, Chung KS, et al. Bawu decoction () ameliorates benign prostatic hyperplasia in rats. Chin J Integr Med. 2017; 23: 611-616.
- Cai H, Zhang G, Yan Z, et al. The Effect of Xialiqi Capsule on Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Evid Based Complement Alternat Med. 2018; 2018: 5367814.
- Gong GY, Xi SY, Li CC, et al. Bushen Tongluo formula ameliorated testosterone propionate-induced benign prostatic hyperplasia in rats. Phytomedicine. 2023; 120: 155048.
- Ma QJ, Gu XQ, Cao X, et al. Effect of beta radiation on TGF-beta1 and bFGF expression in hyperplastic prostatic tissues. Asian J Androl. 2005; 7: 49-54.
- Hennenberg M, Schreiber A, Ciotkowska A, et al. Cooperative effects of EGF, FGF, and TGF-β1 in prostate stromal cells are different from responses to single growth factors. Life Sci. 2015; 123: 18-24.
- Sun H, Li TJ, Sun LN, et al. Inhibitory effect of traditional Chinese medicine Zi-Shen Pill on benign prostatic hyperplasia in rats. J Ethnopharmacol. 2008; 115: 203-208.
- Fruman DA, Chiu H, Hopkins BD, et al. The PI3K Pathway in Human Disease. Cell. 2017; 170: 605-635.
- Zhao F, Zhang CH, Yan JF, et al. Effects of Qianlie Tongqiao Capsule on Bladder Weight and Growth Factors in Bladder Tissue of Rats with Testosterone-Induced Benign Prostatic Hyperplasia. Evid Based Complement Alternat Med. 2018; 2018: 5059267.
- Yan J, Xie Y, Si J, et al. Crosstalk of the Caspase Family and Mammalian Target of Rapamycin Signaling. Int J Mol Sci. 2021; 22.
- Pagano E, Laudato M, Griffo M, et al. Phytotherapy of benign prostatic hyperplasia. A minireview. Phytother Res. 2014; 28: 949-955.
- Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019; 20: 175-193.
- Hosseini ES, Nikkhah M, Hamidieh AA, et al. The Lumiptosome, an engineered luminescent form of the apoptosome can report cell death by using the same Apaf-1 dependent pathway. J Cell Sci. 2020; 133.
- Zhong W, Peng J, He H, et al. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med. 2008; 31: E8-e15.
- Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 2016; 42: 63-71.
- Park E, Lee MY, Seo CS, et al. Yongdamsagan-tang, a traditional herbal formula, inhibits cell growth through the suppression of proliferation and inflammation in benign prostatic hyperplasia epithelial-1 cells. J Ethnopharmacol. 2017; 209: 230-235.
- Liu Y, Shao R, Suo T, et al. Traditional Chinese Medicine Danzhi qing’e decoction inhibits inflammation-associated prostatic hyperplasia via inactivation of ERK1/2 signal pathway. J Ethnopharmacol. 2023; 309: 116354.
- Yuan Y, Yang J, Zhu W, et al. Qianlongtong Inhibits Proliferation and Induces Apoptosis of Hyperplastic Prostate Cells. Am J Mens Health. 2018; 12: 1548-1553.
- Liu L, Wan Y, Shen A, et al. miRNA Regulation Network Analysis in Qianliening Capsule Treatment of Benign Prostatic Hyperplasia. Evid Based Complement Alternat Med. 2015; 2015: 365484.
- Sharma H, Chauhan P, Singh S. Evaluation of the anti-arthritic activity of Cinnamomum cassia bark extract in experimental models. Integr Med Res. 2018; 7: 366-373.
- Latil A, Pétrissans MT, Rouquet J, et al. Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate. 2015; 75: 1857-1867.
- Kij A, Kus K, Czyzynska-Cichon I, et al. Development and validation of a rapid, specific and sensitive LC-MS/MS bioanalytical method for eicosanoid quantification - assessment of arachidonic acid metabolic pathway activity in hypertensive rats. Biochimie. 2020; 171-172: 223-232.
- Liu Y, Duan C, Chen H, et al. Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol Appl Pharmacol. 2018; 351: 1-11.
- Park E, Lee MY, Jeon WY, et al. Inhibitory Effect of Yongdamsagan-Tang Water Extract, a Traditional Herbal Formula, on Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Evid Based Complement Alternat Med. 2016; 2016: 1428923.
- Zhang L, Fan XR, Xie H, et al. Anti-Inflammatory and Antioxidant Effects of Kelong-Capsule on Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Evid Based Complement Alternat Med. 2018; 2018: 5290514.
- Lihua J, Haodan K, Yuan XU. Efficacy of Buzhong Yiqi decoction on benign prostatic hyperplasia and its possible mechanism. J Tradit Chin Med. 2023; 43: 533-541.
- Kim HJ, Park JW, Cho YS, et al. Pathogenic role of HIF-1α in prostate hyperplasia in the presence of chronic inflammation. Biochim Biophys Acta. 2013; 1832: 183-194.
- Zhang J, Zhang X, Xie F, et al. The regulation of TGF-β/SMAD signaling by protein deubiquitination. Protein Cell. 2014; 5: 503-517.
- Chen W, Huang X, Peng A, et al. Kangquan Recipe Regulates the Expression of BAMBI Protein via the TGF-β/Smad Signaling Pathway to Inhibit Benign Prostatic Hyperplasia in Rats. Evid Based Complement Alternat Med. 2019; 2019: 6281819.
- Chen WF, Yang ZB, Peng AX, et al. Effect of Kangquan Recipe () on BAMBI Expression in Hypothalamic-Pituitary-Prostate in Rats with Benign Prostatic Hyperplasia. Chin J Integr Med. 2021; 27: 361-368.
- Zheng H, Xu W, Lin J, et al. Qianliening capsule treats benign prostatic hyperplasia via induction of prostatic cell apoptosis. Mol Med Rep. 2013; 7: 848-854.
- Lin J, Zhou J, Xu W, et al. Qianliening capsule inhibits benign prostatic hyperplasia angiogenesis via the HIF-1α signaling pathway. Exp Ther Med. 2014; 8: 118-124.
- Zhou J, Lin J, Liu L, et al. Qianliening capsules influence the apoptosis of benign prostatic hyperplasia epithelial-1 cells by regulating the extracellular matrix. Mol Med Rep. 2015; 11: 3734-3740.
- Lin J, Zhou J, Zhong X, et al. Inhibition of the signal transducer and activator of transcription 3 signaling pathway by Qianliening capsules suppresses the growth and induces the apoptosis of human prostate cells. Mol Med Rep. 2015; 11: 2207-2214.
- Hong ZF, Lin JM, Zhong XY, et al. Qianliening capsule () inhibits human prostate cell growth via induction of mitochondrion-dependent cell apoptosis. Chin J Integr Med. 2012; 18: 824-830.
- Zhong X, Lin J, Zhou J, et al. Anti-proliferative effects of qianliening capsules on prostatic hyperplasia in vitro and in vivo. Mol Med Rep. 2015; 12: 1699-1708.
- Zang L, Tian F, Yao Y, et al. Qianliexin capsule exerts anti-inflammatory activity in chronic non-bacterial prostatitis and benign prostatic hyperplasia via NF-κB and inflammasome. J Cell Mol Med. 2021; 25: 5753-5768.
- Zang L, Zhang Y, Zhao J, et al. A metabolomics study of Qianliexin capsule treatment of benign prostatic hyperplasia induced by testosterone propionate in the rat model. Anal Biochem. 2021; 628: 114258.
- Huai J, Zhao X, Wang S, et al. Characterization and screening of cyclooxygenase-2 inhibitors from Zi-shen pill by affinity ultrafiltration-ultra performance liquid chromatography mass spectrometry. J Ethnopharmacol. 2019; 241: 111900.
- Wang S, Huai J, Shang Y, et al. Screening for natural inhibitors of 5-lipoxygenase from Zi-shen pill extract by affinity ultrafiltration coupled with ultra-performance liquid chromatography-mass spectrometry. J Ethnopharmacol. 2020; 254: 112733.
- Kim EH, Larson JA, Andriole GL. Management of Benign Prostatic Hyperplasia. Annu Rev Med. 2016; 67: 137-151.
- Foo KT. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)? World J Urol. 2019; 37: 1293-1296.