SciBase Journals
SciBase Surgery
ISSN 2996-590X
- Article Type: Case Report
- Volume 2, Issue 1
- Received: Jan 20, 2024
- Accepted: Feb 26, 2024
- Published Online: Mar 05, 2024
Unnecessary Extended Surgical Resection for Xanthogranulomatous Cholecystitis Mimicking Gallbladder Carcinoma: A Case Report
Hamza Ouzzaouit1,2*; Boubker Idrissi kaitouni1,2; Talha Laalou1,2; Sekkat Hamza1,2; Driss El Alaoui3,2; Zakiya Bernoussi3,2; Abdelmalek Hrora1,2; Mouna Mhamdi Alaoui1,2
1Digestive Surgical Department C, Ibn Sina University Hospital, Rabat, Morocco.
2Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco.
3Anatomopathology Department, Ibn Sina University Hospital, Rabat, Morocco.
*Corresponding Author: Hamza Ouzzaouit
Digestive Surgical Department C, Ibn Sina University Hospital, Rabat, Morocco.
Tel: +212-662-199-277;
Email: hamza_ouzzaouit@um5.ac.ma
Abstract
The Xanthogranulomatous Cholecystitis (XGC) is frequently misdiagnosed as Gallbladder Carcinoma (GBC) and usually undergoes an extended radical surgery. Sometimes XGC might have a coexistent GBC. The dilemma still exists concerning the differential diagnosis in clinical practice, and the final diagnosis has to be dependent on the histological examination. This case shows us the diagnostic difficulty of XGC pre-operatively and the resemblance, especially radiological, with GBC, which led to unnecessary extended surgical resection surgery.
Citation: Ouzzaouit H, kaitouni BI, Laalou T, Hamza S, Alaoui DE, et al. Unnecessary Extended Surgical Resection for Xanthogranulomatous Cholecystitis Mimicking Gallbladder Carcinoma: A Case Report. SciBase Surg. 2024; 2(1): 1007.
Introduction
Xanthogranulomatous Cholecystitis (XGC) is a rare variant of chronic cholecystitis characterized by severe proliferative fibrosis with infiltration of macrophages and foamy cells within the gallbladder wall. This condition is benign in nature but often shows a destructive inflammatory process [1-3]. The inflammatory infiltration causes the asymmetrical thickening of the gallbladder wall and the formation of nodules, which often extend into the neighboring organs [2,3]. The XGC is frequently misdiagnosed as Gallbladder Carcinoma (GBC) and usually undergoes an extended radical surgery [4,5]. Sometimes XGC might have a coexistent GBC [6]. The dilemma still exists concerning the differential diagnosis in clinical practice, and the final diagnosis has to be dependent on the histological examination. This case shows us the diagnostic difficulty of XGC pre-operatively and the resemblance, especially radiological, with GBC, which led to Unnecessary extended surgical resection.
Case presentation
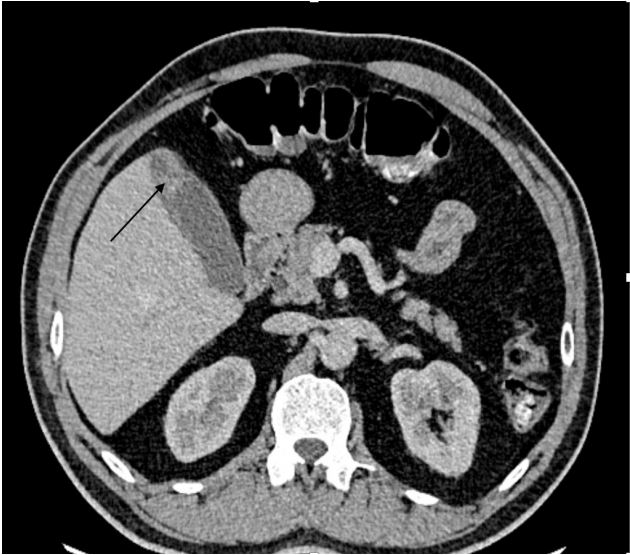
A 58-year-old man with no prior medical history was admitted due to progressively increasing upper abdominal discomfort, lately associated with nausea, vomiting and diarrhea. Clinical examination, found a non-icteric patient, with abdominal pain more marked in the right hypochondrium. His BMI was correct at 28 kg/m. Ultrasound and injected CT scan showed a gallbladder of normal size, with irregular fundic thickening and late enhancement after injection of contrast, measured 15 mm and extending over approximately 2 cm. It is associated with densification of perivesicular fat, with no clear adjacent hepatic invasion. No gallstone was found and no dilation of intrahepatic or extrahepatic bile ducts. Radiological images revealed as well 3 nodes in the hepatic hilum and omental foramen, enhanced after injection of contrast medium, the largest measuring 11x10 mm (Figure 1). The decision of the multidisciplinary consultation meeting was to operate on the patient for gallbladder cancer.
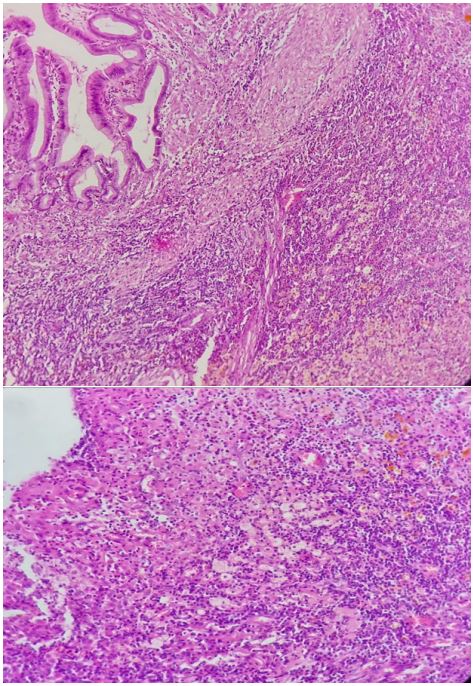
Surgical exploration confirmed the diagnostic findings: The mass completely involved the hepatic hilum, the transverse colon and the second duodenal portion. Assuming an advanced gallbladder carcinoma, we performed the Glenn procedure consisting of a cholecystectomy associated with hepatic resection of the vesicular bed and curage of the hepatic hilum. Histological examination of the surgical specimen showed a fibrous reaction with polymorphous inflammatory granuloma rich in histiocytic cells and the absence of any sign of malignancy, which was in favor of xanthogranulomatous and lithiasis cholecystitis with inflammatory nodes of the hepatic curage (Figure 2). Postoperative period proceeded smoothly, the patient was discharged 5 days after the surgery. Currently, 12 months after surgery, the patient is showing no sign of local recurrence.
Discussion
Xanthogranulomatous cholecystitis is a rare form of chronic cholecystitis that can mimic gallbladder cancer clinically, radiologically and macroscopically. Xanthogranulomatous cholecystitis was first described by McCoy et al. in 1976 [1]. It accounts for 1 to 2% of all cholecystitis [2,3]. XGC predominantly affects patients aged 60 to 70 years, with no gender or racial differences [4]. Xanthogranulomatous inflammation is a destructive inflammatory process that may be chronic, focal, or diffuse. It is histologically characterized by a large number of histiocytes and acute inflammatory cells [7]. The pathogenesis of XGC is not fully understood till now. The presence of gallstones and biliary obstruction might play an important role, which causes the extravasation of the bile into the gallbladder wall via ruptured Rokitansky-Aschoff sinuses and ulcers of the surface mucosa [2,3].
Preoperative diagnosis remains the greatest challenge for surgeons in order to form the best curative plan and avoid useless extensive operations. The close similarities between XGC and GBC may be responsible for more than 1 in 10 patients being treated with unnecessary extended resection or having a missed cancer, as in our patient’s case. In such cases, it is important to carefully evaluate the clinical symptoms and radiological features of XGC in order to avoid unnecessary radical surgery [2]. To our knowledge no specialized studies have been performed concerning the role of tumor markers for this differentiation. The data generated in the series of Zhang et al. did not establish statistical significance for CA19-9. CEA and CA125 are not tumor markers specific for hepatobiliary neoplasms, although statistically significant correlations were found between the two groups [8]. Unfortunately, the clinical indications of XGC are very similar to acute or chronic cholecystitis, and there are no specific signs or symptoms in the differential diagnosis [9].
Although the radiological findings of XGC bear much resemblance to GBC in terms of gallbladder wall thickening and the tendency to involve neighboring organs, the presence of gallstones is very high in XGC cases, compared to GBC. In XGC, it is important to observe the thickening of the gallbladder wall and the presence of gallstones or sludge [10], our patient has no gallstones. Chun et al. [11] found that submucosal hypo attenuated nodules occupying a large area of the thickened gallbladder wall on CT images are highly suggestive of XGC. Moreover, Kim et al. [12] indicated that submucosal nodules on CT images represent abscesses or xanthogranulomas of the gallbladder wall, meaning that the submucosal nodules on the CT images directly reflect the pathological features of XGC, and this accounts for the diagnostic specificity according to CT analysis European studies have reported the incidence of gallstones in XGC to vary between 92% and 100% [2] Uchiyama et al. reported that diffuse wall thickening and intramural nodule formation in the gallbladder on USG were pathognomonic for XGC [13].
In XGC, the thickening of the gallbladder wall is the most common CT finding. Gallstones can be identified on CT. The gallbladder mucosa shows homogeneous contrast enhancement. Similar findings are observed in MRI. However, neither CT or MRI is a specific examination to diagnose XGC [14]. Operative findings commonly reveal the presence of prolific adhesions to surrounding tissues, thick-walled gallbladder often with fistulous communications, gallbladder perforations and abscess formation, resulting in technical difficulties and prolonged operating time [2,10]. When there is a doubt between a XGC and GBC, as Workup, a CT scan, MRI, ideally a radiological or endoscopic guided biopsy and tumor markers should be dosed before and after surgery. The surgery is indicated as soon as GBC workup is completed. In the meanwhile, antibiotic therapy is prescribed [19].
In the study by Hale et al., when there was any doubt between XGC and GBC, the accuracy of performing the correct operation (calculated for 11 studies by the frequency of missed or incorrect diagnosis of GBC) was 338/376 (89,9%). Of 38 patients undergoing an incorrect treatment, 33 patients (8,8%) underwent unnecessary extended resection for suspected GBC while five patients (1.3%), suspected incorrectly to have only XGC without GBC, underwent simple cholecystectomy [2]. GBC cannot be excluded when there is liver or adjacent organ infiltration. Despite the possibility of overtreatment of a substantial proportion patient, most teams recommend total cholecystectomy via open approach with en bloc resection of involved adjacent organs (liver, duodenum, pancreas, colon, omentum, or extrahepatic bile ducts), combined with lymph node dissection of the hepatic pedicle, performed either first-line or after frozen section investigation [20].
Large cohort studies have shown that the general prognosis and survival of advanced GBC are very poor (median 5.8 months; 5-year survival ranging from 0 to 4%), and that radical surgery (with or without biliary resection) does not impact survival in patients with stage III or IV disease [21]. In sum, when there is diagnostic doubt, the principles of GBC surgery should be applied according to local practice, adapting the management to each patient. The postoperative complication rate was noted to be higher in patients of XGC with 10.7% in partial cholecystectomy and 2.8% in total cholecystectomy [18,19]. The average hospital stay was reported to be 21 days (range, 9-60 days) for open cholecystectomy as opposed to 5 days (range, 3-10 days) for Laparoscopic Cholecystectomy [20].
Conclusion
XGC should be taken into account in the differential diagnosis when a suspicious malignant mass is detected in the gallbladder. Although XGC is not a malignant pathology, early diagnosis and treatment are very important due to complications that may occur in the future, and its possible coexistence with GBC. We consider that as the experience of both the surgeon and the radiologist increases in the differentiation of XGC and GBC cases, a healthier differential diagnosis can be made and unnecessary interventions can be avoided.
Declarations
Data availability statement: The data that support the findings of patient information are available from the corresponding author upon reasonable request.
Conflicts of interest: The authors have no conflicts of interest and source of funding. The subject of study had no commercial interest, no financial or material support.
Funding: The authors have no source of funding or financial support except themselves.
Acknowledgments: None.
References
- McCoy JJ Jr, Vila R, Petrossian G, McCall RA, Reddy KS. Xanthogranulomatous cholecystitis. Report of two cases. J S C Med Assoc. 1976; 72(3): 78-9. PMID: 1063276.
- Hale MD, Roberts KJ, Hodson J, Scott N, Sheridan M, Toogood GJ. Xanthogranulomatous cholecystitis: a European and global perspective. HPB (Oxford). 2014; 16(5): 448-58. doi: 10.1111/hpb.12152. Epub 2013 Aug 29. PMID: 23991684; PMCID: PMC4008163.
- Kwon AH, Matsui Y, Uemura Y. Surgical procedures and histopathologic findings for patients with xanthogranulomatous cholecystitis. J Am Coll Surg. 2004; 199(2): 204-10. doi: 10.1016/j.jamcollsurg.2004.03.018. PMID: 15275874.
- Pinocy J, Lange A, König C, Kaiserling E, Becker HD, Kröber SM. Xanthogranulomatous cholecystitis resembling carcinoma with extensive tumorous infiltration of the liver and colon. Langenbecks Arch Surg. 2003; 388(1): 48-51. doi: 10.1007/s00423-003-0362-x. Epub 2003 Mar 20. PMID: 12690480.
- Spinelli A, Schumacher G, Pascher A, Lopez-Hanninen E, AlAbadi H, Benckert C, Sauer IM, Pratschke J, Neumann UP, Jonas S, Langrehr JM, Neuhaus P. Extended surgical resection for xanthogranulomatous cholecystitis mimicking advanced gallbladder carcinoma: A case report and review of literature. World J Gastroenterol. 2006; 12(14): 2293-6. doi: 10.3748/wjg.v12.i14.2293. PMID: 16610041; PMCID: PMC4087666.
- Kwon AH, Sakaida N. Simultaneous presence of xanthogranulomatous cholecystitis and gallbladder cancer. J Gastroenterol. 2007; 42(8): 703-4. doi: 10.1007/s00535-007-2072-6. Epub 2007 Aug 24. PMID: 17701136.
- Roels K, Bogaert J, Van Hoe L. et al. Xanthogranulomatous cholecystitis associated with a xanthogranulomatous pseudotumour on the left diaphragm. Eur Radiol. 1999; 9: 1139-1141. https://doi.org/10.1007/s003300050808.
- Zhang LF, Hou CS, Liu JY, Xiu DR, Xu Z, Wang LX, Ling XF. Strategies for diagnosis of xanthogranulomatous cholecystitis masquerading as gallbladder cancer. Chin Med J (Engl). 2012; 125(1): 109-13. PMID: 22340475.
- Yang T, Zhang BH, Zhang J, Zhang YJ, Jiang XQ, Wu MC. Surgical treatment of xanthogranulomatous cholecystitis: experience in 33 cases. Hepatobiliary Pancreat Dis Int. 2007; 6(5): 504-8. PMID: 17897914.
- Srinivas GN, Sinha S, Ryley N, Houghton PW. Perfidious gallbladders - a diagnostic dilemma with xanthogranulomatous cholecystitis. Ann R Coll Surg Engl. 2007; 89(2): 168-72. doi: 10.1308/003588407X155833. PMID: 17346415; PMCID: PMC1964568.
- Chun KA, Ha HK, Yu ES, Shinn KS, Kim KW, Lee DH, Kang SW, Auh YH. Xanthogranulomatous cholecystitis: CT features with emphasis on differentiation from gallbladder carcinoma. Radiology. 1997; 203(1): 93-7. doi: 10.1148/radiology.203.1.9122422. PMID: 9122422.
- Kim PN, Lee SH, Gong GY, Kim JG, Ha HK, Lee YJ, Lee MG, Auh YH. Xanthogranulomatous cholecystitis: radiologic findings with histologic correlation that focuses on intramural nodules. AJR Am J Roentgenol. 1999; 172(4): 949-53. doi: 10.2214/ajr.172.4.10587127. PMID: 10587127.
- Uchiyama, K., Ozawa, S., Ueno, M. et al. Xanthogranulomatous cholecystitis: the use of preoperative CT findings to differentiate it from gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009; 16: 333-338. https://doi.org/10.1007/s00534-009-0067-9.
- Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004; 8(1): 90-7. doi: 10.1016/j.gassur.2003.10.003. PMID: 14746840.
- Baiu I, Visser B. Gallbladder Cancer. JAMA. 2018; 320(12): 1294. doi: 10.1001/jama.2018.11815. PMID: 30264121.
- Guzmán-Valdivia G. Xanthogranulomatous cholecystitis: 15 years’ experience. World J Surg. 2004; 28(3): 254-7. doi: 10.1007/s00268-003-7161-y. Epub 2004 Feb 17. PMID: 14961199.
- Houston JP, Collins MC, Cameron I, Reed MW, Parsons MA, Roberts KM. Xanthogranulomatous cholecystitis. Br J Surg. 1994; 81(7): 1030-2. doi: 10.1002/bjs.1800810735. PMID: 7922056.
- Parra JA, Acinas O, Bueno J, Güezmes A, Fernández MA, Fariñas MC. Xanthogranulomatous cholecystitis: clinical, sonographic, and CT findings in 26 patients. AJR Am J Roentgenol. 2000; 174(4): 979-83. doi: 10.2214/ajr.174.4.1740979. PMID: 10749233.
- X. Giudicelli, A. Rode, B. Bancel, A.-T. Nguyen, J.-Y. Mabrut, Xanthogranulomatous cholecystitis: Diagnosis and management,Journal of Visceral Surgery. 2021; 158(4): 326-336,1878-7886. https://doi.org/10.1016/j.jviscsurg.2021.02.004.
- Neychev V, Ivanova V, Dikov T, Todorov G. Diffuse xan- thogranulomatous cholecystitis: master of disguise. Cureus 2018; 10(4): 2492.
- Sternby Eilard M, Lundgren L, Cahlin C, Strandell A, Svan- berg T, Sandström P. Surgical treatment for gallbladder cancer — a systematic literature review. Scand J Gastroenterol. 2017; 52(5): 505-14.