SciBase Journals
SciBase Surgery
ISSN 2691-7785
- Article Type: Short Commentary
- Volume 2, Issue 1
- Received: Apr 10, 2024
- Accepted: May 23, 2024
- Published Online: May 30, 2024
Surgical Procedures for the Non-Surgeon: A Rheumatology Perspective
Robert W Ike1*; Kenneth C Kalunian2
1Emeritus Associate Professor, Department of Internal Medicine, Division of Rheumatology, University of Michigan Health System Ann Arbor, Michigan, USA.
2Professor, Department of Medicine, Division of Rheumatology, Allergy, and Immunology, University of California, San Diego, USA.
*Corresponding Author: Robert W Ike
Emeritus Associate Professor, Department of Internal Medicine, Division of Rheumatology, University of Michigan Health System, Ann Arbor, Michigan, USA.
Email: scopydoc52@yahoo.com
Abstract
Each specialty of medicine (and surgery) employs certain technical procedures appropriate to their specialty. Occasionally, those procedures require performance in an operating room environment. With the O.R. considered the province of the surgeon, such trespass by non-surgeons is not always well accepted. Rheumatologists seeking to develop arthroscopy as a tool for arthritis patients encountered such resistance but were able to persist and now perform any arthroscopy exclusively in a clinic or procedure room setting. Other procedures now performed by rheumatologists - arthrocentesis, joint washout, synovial biopsy, and labial salivary gland biopsy (lip biopsy) -all had their beginnings in the operating room, but no longer require such an environment. Non-surgeons - including rheumatologists - seeking to develop new invasive procedures may continue to utilize the O.R. in early stages when caution and safety concerns demand high level support, and sterile technique. The history of development of these procedures shows a common path to bedside performance. Any O.R. turf touched by a non-surgeon is only temporary.
Citation: Ike RW, Kalunian KC. Surgical Procedures for the Non-Surgeon: A Rheumatology Perspective. SciBase Surg. 2024; 2(1): 1010.
Introduction
Do you have to be a surgeon to do a procedure in the operating room? And does the non-surgeon performing that procedure in the O.R. bring a different perspective to the process? The paths that rheumatologists have had to take with several of the procedures they employ shed some light on these issues.
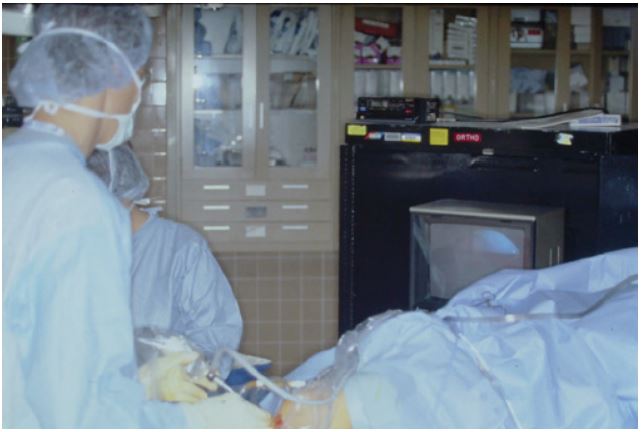
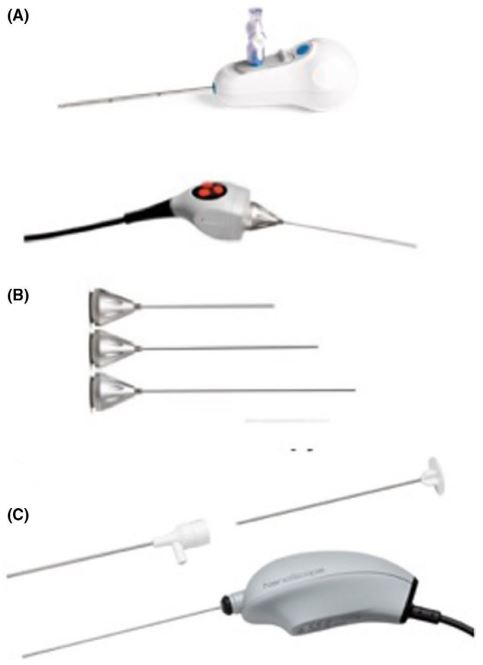
Arthroscopy: When some rheumatologists took a serious interest in arthroscopy in the 1980s [1], the operating room, with sterile technique and anesthesia, was the only venue in which the procedure could be performed [2]. Despite documentation of adequate training and competence in the procedure, interested rheumatologists had difficulty entering this environment [3]. These barriers eventually fell, and rheumatologists safely and effectively performed arthroscopy on their arthritis patients for a couple of decades (Figure 1). Technical evelopments with the arthroscope made it possible to perform the procedure in outpatient settings away from the operating room, such as procedure rooms, or even the clinic. Use of arthroscopy for therapeutic applications faded when controlled trials found no benefit for arthroscopic interventions over placebo in osteoarthritis [4] and efficacy of biologic treatments substantially reduced the indications for arthroscopic interventions in inflammatory arthropathies [5]. Arthroscopy remains a valuable tool for assessment of synovial disorders, particularly important as rheumatology enters a phase of “precision” medicine, with treatments tailored to synovial features. But all such procedures are performed in procedure rooms or at the bedside. A new generation of “nano scopes” (Figure 2) makes in-office arthroscopy all the more feasible. Some orthopedists have recognized this [6]. Rheumatologists interested in using arthroscopy for research purposes are attracted to these much smaller ‘scopes [7].
Arthrocentesis: All of the many technical procedures performed by rheumatologists had their beginnings in the operating room. In the early days of arthrocentesis, concern for consequences of violating the joint integrity had the tap done in the operating room, under sterile conditions [8]. While attention to asepsis remains an important component of bedside arthrocentesis, this most common of rheumatologic procedures is carried out around the world with only very rare complications [9].
Washout: Joint puncture with intent to duplicate what might be accomplished with the arthroscope has spawned two other bedside procedures. The first is joint washout. Improvement of arthritis symptoms after arthroscopy, attributed to joint distension and irrigation necessary to provide a clear view, was observed by some of the first practitioners of arthroscopy shortly after the turn of the last century [10]. The father of modern arthroscopy, Makei Watanabe, after WWII made a dedicated effort to duplicate that washout effect without having to go to the O.R [11]. The washout technique has had a checkered history since, although compelling evidence has been accumulated suggesting that washout can be a useful adjunct in managing degenerative, inflammatory, crystalline, and infectious processes affecting the knee [12]. For a patient with a septic knee, an urgent trip to the operating room for arthroscopic washout is still standard of care [13]. With that washout duplicated by bedside puncture, that trip may not always be necessary [14].
Synovial biopsy: Much of arthroscopy’s appeal to rheumatologists rests in the excellent view of macroscopic pathology afforded [15] and the possibility to guide sampling of this tissue. The synovium is the target tissue for the many immunemediated processes that can affect joints and reaction of that tissue to processes that originate elsewhere, like osteoarthritis and crystalline arthropathies, can provide insight onto those entities. With only a few rheumatologists still proficient at arthroscopy, other means of sampling synovium have been developed. “Blind” bedside biopsy was once an important clinical tool prior to the introduction of the polarizing light microscope when crystalline arthropathies could only be diagnosed by their tissue characteristics [16]. A renaissance in interest in synovial biopsy has risen from the notion that synovial characteristics can be used to classify disease, guide treatment, and monitor response to that treatment. Optimistic talk of “precision medicine” has emerged out of the notion that target tissue features can guide specific treatments [17,18]. Emergence of bedside ultrasound as a widely mastered skill [19] has helped focus bedside biopsy on visualizable tissue pathology. Feasibility of a coordinated multicenter approach to ultrasound guided assessment of synovium in early arthritis has been demonstrated in a large trial [20]. Appreciation of synovial characteristics, and volume of tissue attainable, remain better served by arthroscopy. Whether rheumatologists return to the arthroscope, in its new “nano” configuration, or stay with ultrasound remains to be determined. Regardless, these investigations take place far away from the operating room.
Muscle biopsy: In perhaps the earliest adaptation to operating room difficulties, at least in the rheumatic diseases, the great student of neuromuscular diseases, Duchenne, developed a percutaneous muscle biopsy instrument when he was excluded from Parisian operating rooms by surgeons who disdained his lowly origins and accent [21]. His design has gone through many modifications over the years [22]. Whether muscle biopsy for assessment of neuromuscular disease should be performed as an open procedure in the OR or maybe procedure room versus percutaneous sampling remains a matter of controversy [23], but the latter makes the intervention accessible to non-surgeons without an operating room. A critical requirement is a pathologist familiar with handling smaller amounts of tissue [24].
1.9 mm disposable scope and camera with 2.2 mm inflow cannula and 120° field of view. https://www.arthrex.com/what-surgeonsare-talking-about/78CC3845-4F4A-4F8A-A867-016B995DFC52. From reference 7, with permission.
*Note that as of this writing, VisionScope Technologies is no longer in business.
Labial salivary gland biopsy: Only very small amounts of salivary gland tissue are needed to support or refute a suspected diagnosis of Sjögren’s disease. While the technique for doing so was developed by an oral surgeon over 40 years ago [25], proficiency in the technique has not been widespread. Clinicians seeking salivary gland tissue confront oral surgeons, otorhinolaryngologists, and dentists for whom the procedure is an afterthought, maybe tacked on to the end of an O.R. schedule. Fortunately, skills to perform this biopsy procedure are within the realm of any rheumatologist capable of sewing up a simple laceration [26]. Rheumatologists interested in acquiring this skill have had a hands-on workshop at a national meeting [27] and access to on-line demonstrations of the technique [28]. Rheumatologists billing for the procedure see a financial bonus, as the CPT code was calculated as if the procedure was done in the O.R. That can’t last. Regardless, rapid access to a diagnostic procedure performed by someone familiar with the entity in question has great value. Besides Sjögren’s disease, there are other entities for which features of salivary gland pathology can be diagnostic or supportive [26].
Conclusion
Several technical procedures are important to the practice of rheumatology and began as “surgical” procedures performed in the operating room. As it became possible to accomplish the goals of these procedures away from the confines and restrictions of the operating room, performance moved to procedure rooms and the clinic bedside. The consequence has been availability of procedures performed by a practitioner well versed in the clinical entities under investigation without need for an external referral. Prompt attention to such needs benefits rheumatologist and patient alike. New, as yet unrealized, procedures in rheumatology may also require initial performance in an operating room. Based on history of previous procedures, such passage will likely be transient [29].
References
- Ike RW, Arnold WJ, Kalunian KC. Arthroscopy in rheumatology. Where have we been? Where might we go? Rheumatol (Oxford). 2021; 60: 518-528.
- Arnold WJ, Kalunian K. Arthroscopic synovectomy by rheumatologists: Time for a new look. Arthritis Rheum. 1989; 32(1): 109-111.
- Ike RW. Who let this flea into my operating room? Clin Med. 2021; 3(3): 1039-1040.
- Katz JN, Brownlee SA, Jones MH. The role of arthroscopy in the management of knee osteoarthritis. Best Pract Res Clin Rheumatol. 2014; 28(1): 143-156.
- Saeki Y, Matsui T, Saisho K, Tohma S. Current treatments of rheumatoid arthritis: from the ‘NinJa’ registry. Expert Rev Clin Immunol. 2012; 8(5): 455-465.
- Zhang K, Crum RJ, Samuelsson K, Cadet E, Ayeni OR, et al. In-office needle arthroscopy: A systematic review of indications and clinical utility. Arthroscopy. 2019; 35(9): 2709-2721.
- Ike RW, Kalunian KC. Will rheumatologists ever pick up the arthroscope again?. Int J Rheum Dis. 2021; 24(10): 1235-1246.
- Kaiser, H. Gelenkpunktion und -injektion - Die Geschichte A History of Joint Puncture and Injection. Z. Rheumatol. 2011; 70: 69-78.
- Robert WN, Hauptman HW. Joint aspiration or injection in adults: Complications. UpToDate. Version. 2024. https://medilib.ir/uptodate/show/7977.
- Burnam MS. Arthroscopy or direct visualization of joints. J Bone Joint Surg. 1931; 13: 669-695.
- Watanabe M. Articular pumping. J Jap. Orthop. Assoc. 1949; 24: 30-42.
- Ike RW, Kalunian KC. Is it time to bring back knee washout? J Rheumatol. 2022; 49(12): 1307-1314.
- Elsissy JG, Liu JN, Wilton PJ, Nwachuku I, Gowd AK, et al. Bacterial Septic Arthritis of the Adult Native Knee Joint: A Review. JBJS Rev. 2020; 8(1): e0059.
- Ike RW. Can bedside knee joint washout help treat septic arthritis? Ann Orthop Rheumatol. 2021; 8(1): 1097 1-4.
- Ike RW. Minimally invasive procedures, in Rheumatology, MC Hochberg, J Silman, JS Smolen, ME Weinblatt, and MH Weisman, editors. Third edition, Harcourt Health Sciences: London. 2003; 245-252.
- Mikkelsen WM, Duff IF, Castor CW, Zeveley HA, French AJ. The diagnostic value of punch biopsy of the knee synovium. AMA Arch Intern Med. 1958; 102(6): 977-985.
- Bhamidipati K, Wei K. Precision medicine in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2022; 36(1): 101742.
- Lewis MJ. Predicting best treatment in rheumatoid arthritis. Semin Arthritis Rheum. 2024; 64S: 152329.
- Cipolletta E, Filippucci E, Incorvaia A, Schettino M, Smerilli G, Di Battista J et al. Ultrasound-Guided Procedures in Rheumatology Daily Practice: Feasibility, Accuracy, and Safety Issues. J Clin Rheumatol. 2021; 27(6): 226-231.
- Donlin LT, Rao DA, Wei K, Slowikowski K, McGeachy MJ, et al. Methods for high-dimensional analysis of cells dissociated from cryopreserved synovial tissue. Arthritis Res Ther. 2018; 20(1): 139.
- Charrière M, Duchenne GB. Emporte pièce histologique Histological cookie cutter. Bull Academ Med. 1865; 1050-1.
- O’Rourke KS, Ike RW. Muscle biopsy. Curr Opin Rheumatol. 1995; 7(6): 462-468.
- Ross L, McKelvie P, Reardon K, Wong H, Wicks I, et al. Muscle biopsy practices in the evaluation of neuromuscular disease: A systematic literature review. Neuropathol Appl Neurobiol. 2023; 49(1): e12888.
- Nelson CC, Blaivas M. Orbicularis oculi muscle in children. Invest Ophthalmol Vis Sci. 1992; 12: 191-196.
- Daniels TE. Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984; 27(2): 147-156.
- Ike RW, McCoy SS. Bedside Labial Salivary Gland Biopsy (LSGBx: Lip Biopsy). An Update for Rheumatologists. (invited review) Best Pract Clin Res Rheum Mar. 2023; 37(1): 101839.
- Bernstein S. Intensive, personalized learning. The Rheumatologist September. 2019.
- Ike RW, McCoy SS. Learn labial salivary gland biopsy online. J Clin Rheumatol October. 2023; 29(7): 363.
- Ike RW, McCoy SM, Kalunian KC. What bedside skills could the modern rheumatologist possess?. Part II. Certain Technical Procedures. J Clin Rheum. 2023.