SciBase Journals
SciBase Surgery
ISSN 2691-7785
- Article Type: Case Report
- Volume 1, Issue 1
- Received: Sep 12, 2023
- Accepted: Jan 24, 2024
- Published Online: Jan 31, 2024
Delayed Composite Mesh Infection: Can Burkholderia Cepacia be Incriminated?
Hana Chaabouni1; Fatma Hammami1*; Fatma Smaoui1; Khaoula Rekik1; Chakib Marrakchi1; Salah Boujelbene2; Makram Koubaa1; Mounir Ben Jemaa1
1Infectious Diseases Department, Hedi Chaker University Hospital, University of Sfax, Tunisia.
2General Surgery Department, Habib Bourguiba Hospital, University of Sfax, Tunisia.
*Corresponding Author: Fatma Hammami
Infectious Diseases Department, Hedi Chaker University Hospital, University of Sfax, Tunisia.
Tel: +216-51-755-665;
Email: fatma.hammami@medecinesfax.org
Abstract
Delayed mesh infection is a rare complication. In this report, we describe the case of a 68-year-old woman who, underwent cholecystectomy and an eventration on a sub umbilical hernia which required composite mesh implantation with ceolioscopy in 2014. Nine years after the hernia repair, she presented with an infected mesh due to Burkholderia Cepacia. This infection required medical and surgical treatment with mesh removal in order to cure the patient. The mechanism and etiology of such a late complication are discussed.
Keywords: Composite mesh; Infection; Burkholderia Cepacia.
Abbreviations: VHR: Ventral Hernia Repair; PTFE: Polytetrafluoroethylene; BCC: Burkholderia Cepacia Complex; PP: Polypropylene.
Citation: Chaabouni H, Hammami F, Smaoui F, Rekik K, Marrakchi C, et al. Delayed Composite Mesh Infection: Can Burkholderia Cepacia be Incriminated?. SciBase Surg. 2024; 2(1): 1006.
Introduction
Delayed mesh infection is a rare complication and the precise mechanism of its development is unknown [1]. Mesh infection, the most devastating mesh-related complication after ventral hernia repair (VHR), may occur in 7% to 10% of patients [2,3]. The most common bacteria associated with prosthetic mesh infection are Staphylococcus aureus (57.7%). Treatment strategies that have been described for mesh infection include complete or partial mesh removal, antimicrobial therapy, and conservative treatment to salvage the mesh [2,4,5]. More recently, management of some patients has shifted to an attempt of conservative treatment (antibiotics, wound management, etc.) to salvage mesh without surgical removal [5]. We report herein a case of composite mesh infection in a 68-year-old patient due to Burkholderia Cepacia and after our review by searching PubMed publications till June 2023, our case is the first case of mesh infection secondary to this pathogen.
Case presentation
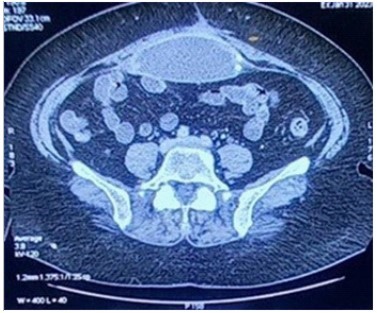
A 68-year-old woman with a previous medical history of high blood pressure and dyslipidemia, presented with a 15- day history of abdominal pain and subocclusif syndrome. She underwent a cholecystectomy in 2013. One year later, she presented an eventration on a sub umbilical hernia which required composite mesh implantation with ceolioscopy in 2014. She remained disease free and asymptomatic from her abdominal incision until 2023 when she developed a subocclusif syndrome and abdominal pain. In january 2023, the patient was admitted with a suspected intra-abdominal cause. On admission, she was apyretic. Abdominal examination revealed a widespread abdominal tenderness, no hepatomegaly, nor splenomegaly. She presented a discrete systolic murmur at the aortic focus. Laboratory investigations revealed elevated C-reactive protein levels at 60 mg/L, a white blood cell count at 4450/mm3 , anemia with an hemoglobin level at 9.9 g/dL, and platelets count at 258000/mm3 . Renal and hepatic functions were normal. Blood and urine cultures were collected on admission. Transthoracic echocardiography was normal. Following admission, abdominal Computed Tomography scan (CT) revealed abcess formation with contrast effect regarding the mesh, on the supraumbilical anterior abdominal wall, measuring 11.7 cm transverse diameter x10.5 cm craniocaudal diameter x4.5 cm thick (Figure 1).
The patient underwent CT-guided percutaneous drainage of the abscess. A drain was placed and a sample was taken and sent for bacteriological study. She was started empiric antibiotic therapy based on imipenem 1 gr twice daily, metronidazole 500 mg 3 times daily and pristinamycin 500 mg 2 tablets 3 times daily. Abcess culture grew Burkholderia Cepacia, which was sensitive to meropnem, minocycline, levofloxacin and cotrimoxazole. The patient had received imipenem combined with levofloxacin 500 mg twice daily and cotrimoxazole 1 pill twice daily. The drainage volume decreased gradually until no fluid was observed. On day 20, the drainage tube was removed.
On day 27 of treatment, the patient had no functional complaints. Laboratory investigations revealed no inflammatory markers. However, abdominal ultrasound showed the persistence of the collection (67x6x84 mm) on the deep side of the mesh.
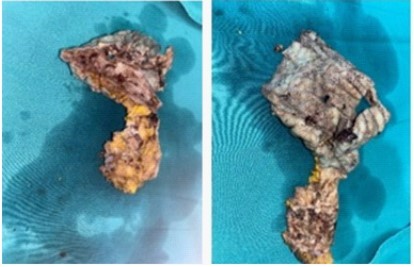
Following the trial of intravenous antibiotics, a multidisciplinary review recommended that the mesh should be removed (Figure 2). She received metronidazol 500 mg 3 times daily and cotrimoxazole 1 pill twice daily for 2 weeks and levofloxacin 500 mg 2 times daily for 3 weeks. After the removal of the mesh, the patient was reviewed four weeks later with no further infections and a well-healed scar
Discussion
We report a case of composite mesh infection in a 68-yearold patient due to Burkholderia Cepacia. We reviewed the literature by searching PubMed publications till June 2023, our case is the first case of mesh infection secondary to this pathogen.
Because of unacceptably high recurrence rates after suturebased VHR, using reinforcing mesh has become a commonly performed and widely accepted procedure [2,6,7]. Although mesh has reduced hernia recurrence rates, it has its own set of complications. Infection is one of the most devastating complications [2,5]. It is associated with hospital readmission, increased healthcare costs, reoperation, hernia recurrence and impaired quality of life [8].
Mesh infection, the most devastating mesh-related complication after VHR, may occur in 7% to 10% of patients [2,3]. The incidence of mesh infections after incisional hernia repair is about 1% for endoscopic techniques and can be more than 15% in other techniques [9]. Several factors have been identified as being significantly associated with mesh infection and included patient-related factors such as diabetes, obesity, smoking, chronic obstructive pulmonary disease, immunosuppressive treatments [10], factors related to the operating conditions such as strangulated hernia, lack of prophylactic antibiotics at induction and factors related to the prosthesis used [5]. In fact, polytetrafluoroethylene (PTFE) and microporous prostheses are more likely to be associated with prosthesis infection [2,11-14].
Usually, mesh infections occur soon after surgery, within weeks or months at the latest. Delayed infections occuring many years after the abdominal wall mesh insertion are extremely uncommon [14]. One of the mechanisms to be considered is the hematogenous spread of infection and late wound breakdown, which would allow its introduction. Another potential or possible cause is percutaneous drainage of a reactive seroma, which may develop around a longstanding foreign body [14]. According to Jose´ Bueno-Lledo et al., the time frame from hernia repair to mesh infection in their series was 10.3 months (range 1 to 29 months) [15]. Indeed, 34.7% of patients presented greater than a year after mesh implantation with a mesh infection [5]. In contrast, A Elfaki et al. reported the longest documented case of mesh infection, occurring over 15 years after mesh insertion [14]. For our patient, the delay was about 9 years after mesh insertion and the mechanism would be probably an hematogenous spread secondary to dental care done two weeks before or an intraoperative revealed very late.
Bacteria typically involved in mesh infection include Staphylococcus aureus, Staphylococcis coagulase negative and enteric Gram negative bacteria [8,16]. In the literature, Staphylococcus aureus is identified in the majority of prosthetic mesh infections, with a rate estimated in different studies at 81%, 57,7% and 42% [8,17]. However, M. Siebert et al. reported that of the 22 documented mesh infections, 3% were polymicrobial. A total of 54 bacteria were isolated, and the most frequently represented bacteria were Enterobacteriaceae (18). For our patient, culture grew Burkholderia Cepacia. In fact, the Burkholderia Cepacia Complex (BCC) is a group of non-fermenting and oxidase-positive aerobic Gram-negative bacilli [19-21]. Burkholderia causes respiratory infections in immunocompromised patients, especially those with cystic fibrosis and chronic granulomatous disease [22]. It also has been reported in immunocompromised patients: debilitated elderly people, HIV-positive individuals, cancer patients undergoing chemotherapy [20,23- 25]. There have also been reports documenting BCC as being responsible for endocarditis in drug addicts or patients with prosthetic heart valves [26], eye infections following surgery [27] and infections or abscesses of the central nervous system [28]. BCC is also rarely responsible for refractory peritonitis in patients undergoing continuous ambulatory peritoneal dialysis [29,30]. Some cases of splenic and hepatic abcesses were also reported [31,32].
After a careful review of the litterture, we didin’t found any case of mesh infection due to Burkholderia Cepacia. So, we reported the first case of Burkholderia Cepacia mesh infection. Current treatment for mesh infections is complex due to numerous variables unique to each patient and hernia including patient factors, surgical technique, degree of contamination, mesh type and position, and wound closure [5].
Concerning mesh type, today’s prosthetics may be classified broadly into three categories: synthetic, composite, and biologic. Synthetic mesh, such as polypropylene (PP) or polyester, is characterized by high tensile strength and vigorous tissue ingrowth, but is unsuitable for intraabdominal placement because of its tendency to induce bowel adhesions. Composite, or barrier-coated, mesh is a dual-sided prosthetic having a synthetic parietal side to promote a strong repair and a visceral surface that repels tissue ingrowth and decreases adhesion formation [33]. Biologic mesh are derived from human, bovine, and porcine tissue that has been decellularized to leave a collagen matrix and then be incorporated into the host tissue. Biological meshes theoretically generate less of a foreign body response and are more resistant to infection [34].
The current recommended treatment for mesh infection remains complete foreign body removal and antibiotic therapy, including complete removal of mesh and sutures with closure of the fascia [5,9,16,34]. In fact, the initial period of bacterial adhesion can be rapid and reversible. However, subsequent irreversible mesh attachment via bacterial adhesins and production of bacterial biofilm impairs penetration and clearance of bacteria by host immune cells and systemic antibiotics [15,35]. Consequently, when an infection is established, this capsule restricts the penetration of antimicrobial agents into the infected mesh [15], that’s why complete removal of mesh is recommended.
More recently, management of some patients has shifted to an attempt of conservative treatment based on antibiotics, wound management and salvage mesh without surgical removal [2,4,5].
In fact, Stremitzer et al. evaluated 31 patients with a mesh infection after VHR and found a mesh salvage rate of 55% with conservative treatment. According to the same study, there was a significant association between the type of mesh graft used and the probability of mesh preservation in case of infection. While conservative therapy led to preservation of 100% of absorbable infected polyglactin/PP meshes, only 20% of infected pure PP meshes and 23% of infected PTFE/PP meshes could be salvaged using conservative means (p<0.0001) [36].
These results are explained in the literature. In fact, in the case of PP meshes, this is most likely due to the pore size inherent in this particular mesh, allowing free flow of bacteria as well as host immune response cells resulting in a more tightly integrated and solid repair [37,38]. On the other hand, PTFE mesh is constructed with very tiny pores, purposefully designed to prevent this free flow of fuid and cells and adhesions. The PTFE layer of this composite type of mesh uniquely creates a diffcult environment to successfully control infection [38,39].
For our patient, she had a composite mesh and several retrospective studies have investigated the incidence of infection after composite mesh repair. In one study, 3.3% of patients undergoing VHR with a composite mesh required mesh explantation because of infection [40].
Greenberg et al reported in their study that eleven patients with composite mesh-related infections, were treated initially with conservative measures. Four patients ultimately required mesh removal. Seven patients, however, were successfully salvaged with conservative methods, including dressing changes, local wound debridement, partial mesh excision, wound vacuum, and antibiotics [38]. None of the patients with a salvaged infected composite mesh developed hernia recurrence in a three-year follow-up. This study stand in contrast to a retrospective review, published by William S Cobb et al. that reported a 10% infection rate among 206 patients who underwent elective composite mesh hernia repair. All but two necessitated mesh removal [3]. Additionally, 95% of heavyweight PPE mesh and all PTFE/PPE meshes have been excised [5]. For our patient mesh excision was due to failure of conservative treatment.
Conclusion
In conclusion, for our patient, the mesh type, the presence of biofilm, failure of conservative treatment (drainage and antibiotics) lead to the mesh excision with a favourable outcome. Each patient must be evaluated and treated on a case-by-case basis. Not all infected composite mesh implants mandate removal. Each patient’s presentation must be individualized. Mesh salvage should be considered if possible before condemning patients to mesh explantation and its inherent morbidity.
Declarations
Conflicts of interest: There is no conflicts of interest to declare..
Funding sources: None.
Acknowledgments: None.
References
- Homare Ito, Kenji Matsumoto, Toshiaki Terauchi, Masaru Kimata, Alan Kawarai Lefor, Hiroharu Shinozaki. Delayed mesh infection after inguinal hernia repair: a case report. J Surg Case Rep. 2021; 22; 2021(9): rjab399. doi: 10.1093/jscr/rjab399.
- Plymale MA, Davenport DL, Walsh-Blackmore S, Hess J, Griffiths WS, Plymale MC, et al. Costs and Complications Associated with Infected Mesh for Ventral Hernia Repair. Surg Infect. 2020; 21(4): 344‑9.
- Cobb WS, Carbonell AM, Kalbaugh CL, Jones Y, Lokey JS. Infection risk of open placement of intraperitoneal composite mesh. Am Surg. 2009; 75(9): 762‑7; discussion 767-8.
- Bueno-Lledó J, Torregrosa-Gallud A, Carreño-Saénz O, GarcíaPastor P, Carbonell-Tatay F, Bonafé-Diana S, et al. Partial versus complete removal of the infected mesh after abdominal wall hernia repair. Am J Surg. 2017; 214(1): 47‑52.
- Kao AM, Arnold MR, Augenstein VA, Heniford BT. Prevention and Treatment Strategies for Mesh Infection in Abdominal Wall Reconstruction. Plast Reconstr Surg. 2018; 142(3 Suppl): 149S155S.
- Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004; 240(4): 578‑83; discussion 583-585.
- R W Luijendijk, W C Hop, M P van den Tol, D C de Lange, M M Braaksma, J N IJzermans, et al. A Comparison of Suture Repair with Mesh Repair for Incisional Hernia | NEJM. 2000; 343(6): 392-8. doi: 10.1056/NEJM200008103430603.
- Wilson RB, Farooque Y. Risks and Prevention of Surgical Site Infection After Hernia Mesh Repair and the Predictive Utility of ACS-NSQIP. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2022; 26(4): 950‑64.
- Dietz UA, Spor L, Germer CT. [Management of mesh-related infections]. Chir Z Alle Geb Oper Medizen. 2011; 82(3): 208‑17.
- Szczerba SR, Dumanian GA. Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg. 2003; 237(3): 437‑41.
- 11 Shankaran V, Weber DJ, Reed RL, Luchette FA. A review of available prosthetics for ventral hernia repair. Ann Surg. 2011; 253(1): 16‑26.
- Cohn SM, Giannotti G, Ong AW, Varela JE, Shatz DV, McKenney MG, et al. Prospective randomized trial of two wound management strategies for dirty abdominal wounds. Ann Surg. 2001; 233(3): 409‑13.
- Van’t Riet M, de Vos van Steenwijk PJ, Bonjer HJ, Steyerberg EW, Jeekel J. Mesh repair for postoperative wound dehiscence in the presence of infection: is absorbable mesh safer than nonabsorbable mesh. Hernia. 2007; 11(5): 409‑13.
- Elfaki A, Gkorila A, Khatib M, Malata CM. Infection of PTFE mesh 15 years following pedicled TRAM flap breast reconstruction:mechanism and aetiology. Ann R Coll Surg Engl. 2018; 100(1): e18‑21.
- Bueno-Lledó J, Torregrosa-Gallud A, Sala-Hernandez A, Carbonell-Tatay F, Pastor PG, Diana SB, et al. Predictors of mesh infection and explantation after abdominal wall hernia repair. Am J Surg. 2017; 213(1): 50‑7.
- Falagas ME, Kasiakou SK. Mesh-related infections after hernia repair surgery. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2005; 11(1): 3‑8.
- Birolini C, Faro Junior MP, Terhoch CB, de Miranda JS, Tanaka EY, Utiyama EM. Microbiology of chronic mesh infection. Hernia. 2023; 27(4): 1017-1023. doi: 10.1007/s10029-023-02747-6.
- Siebert M, Lhomme C, Carbonnelle E, Trésallet C, Kolakowska A, Jaureguy F. Épidémiologie microbienne et sensibilité aux antibiotiques des prothèses pariétales abdominales infectées. J Chir Viscérale. 2023; 160(2): 91‑6.
- Sfeir MM. Burkholderia Cepacia complex infections: More complex than the bacterium name suggest. J Infect. 2018; 77(3): 166‑70.
- Tavares M, Kozak M, Balola A, Sá-Correia I. Burkholderia Cepacia Complex Bacteria: a Feared Contamination Risk in WaterBased Pharmaceutical Products. Clin Microbiol Rev. 2020; 33(3): e00139-19.
- Häfliger E, Atkinson A, Marschall J. Systematic review of healthcare-associated Burkholderia Cepacia complex outbreaks: presentation, causes and outbreak control. Infect Prev Pract. 2020; 2(3): 100082.
- Martin DW, Mohr CD. Invasion and intracellular survival of Burkholderia Cepacia. Infect Immun. 2000; 68(1): 24‑9.
- El Chakhtoura NG, Saade E, Wilson BM, Perez F, Papp-Wallace KM, Bonomo RA. A 17-Year Nationwide Study of Burkholderia Cepacia Complex Bloodstream Infections Among Patients in the United States Veterans Health Administration. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017; 65(8): 1253‑9.
- Singhal T, Shah S, Naik R. Outbreak of Burkholderia Cepacia complex bacteremia in a chemotherapy day care unit due to intrinsic contamination of an antiemetic drug. Indian J Med Microbiol. 2015; 33(1): 117‑9.
- Mann T, Ben-David D, Zlotkin A, Shachar D, Keller N, Toren A, et al. An outbreak of Burkholderia cenocepacia bacteremia in immunocompromised oncology patients. Infection. 2010; 38(3): 187‑94.
- Dellalana LE, Byrge KC, Gandelman JS, Lines T, Aronoff DM, Person AK. A Unique Case of Burkholderia Cepacia Prosthetic Mitral Valve Endocarditis and Literature Review. Infect Dis Clin Pract Baltim Md. 2019; 27(3): 123‑5.
- Sachdeva V, Pathengay A, Joseph J, Sharma S, Das T. Burkholderia Cepacia endophthalmitis: clinico-microbiologic profile and outcomes. Retina Phila Pa. 2011; 31(9): 1801‑5.
- Kwayess R, Al Hariri HE, Hindy JR, Youssef N, Haddad SF, Kanj SS. Burkholderia Cepacia Infections at Sites Other than the Respiratory Tract: A Large Case Series from a Tertiary Referral Hospital in Lebanon. J Epidemiol Glob Health. 2022; 12(3): 274‑80.
- Huang X, Yang T, Li M, Wang C, Zhou Y, Zhang J. Burkholderia Cepacia-A rare but important cause of refractory peritonitis in patients with continuous ambulatory peritoneal dialysis: A case report and literature review. Semin Dial. 2022; 35(2): 190‑3.
- Hamahata A, Mitsusada S, Iwata T, Nakajima K, Ogawa Y, Miyazaki A, et al. Liver Cirrhosis Complicated by Spontaneous Bacterial Peritonitis Caused by the Burkholderia Cepacia Complex. Intern Med Tokyo Jpn. 2021; 60(21): 3435‑40.
- Iyer RN, Jangam RR, Nara BK, Kondeti KA. Multiple hepatic and splenic abscesses due to Burkholderia pseudomallei. Indian J Med Microbiol. 2021; 39(2): 249‑51.
- Nittala R, Behera MK, Panigrahy R, Narayan J, Mishra D, Singh A, et al. Burkholderia Cepacia causing liver and splenic abscess: Two case reports. Indian J Pathol Microbiol. 2023; 66(1): 171‑3.
- Jacob BP, Hogle NJ, Durak E, Kim T, Fowler DL. Tissue ingrowth and bowel adhesion formation in an animal comparative study: polypropylene versus Proceed versus Parietex Composite. Surg Endosc. 2007; 21(4): 629‑33.
- FitzGerald JF, Kumar AS. Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons? Clin Colon Rectal Surg. 2014; 27(4): 140‑8.
- J F Gillion, J P Palot. Éventrations. Prothèses infectées : traitement et prévention. EM-Consulte. Available at: https: //www.em-consulte.com/article/768977/eventrations-protheses-infectees-traitement-et-pre.
- Topart P, Ferrand L, Vandenbroucke F, Lozac’h P. Laparoscopic ventral hernia repair with the Goretex Dualmesh: long-term results and review of the literature. Hernia J Hernias Abdom Wall Surg. 2005; 9(4): 348‑52.
- Stremitzer S, Bachleitner-Hofmann T, Gradl B, Gruenbeck M, Bachleitner-Hofmann B, Mittlboeck M, et al. Mesh graft infection following abdominal hernia repair: risk factor evaluation and strategies of mesh graft preservation. A retrospective analysis of 476 operations. World J Surg. 2010; 34(7): 1702‑9.
- Hanna M, Dissanaike S. Mesh ingrowth with concomitant bacterial infection resulting in inability to explant: a failure of mesh salvage. Hernia J Hernias Abdom Wall Surg. 2015; 19(2): 339‑44.
- Greenberg JJ. Can infected composite mesh be salvaged? Hernia J Hernias Abdom Wall Surg. 2010; 14(6): 589‑92.
- Paton BL, Novitsky YW, Zerey M, Sing RF, Kercher KW, Heniford BT. Management of infections of polytetrafluoroethylene-based mesh. Surg Infect. 2007; 8(3): 337‑41.
- Iversen E, Lykke A, Hensler M, Jorgensen LN. Abdominal wall hernia repair with a composite ePTFE/polypropylene mesh: clinical outcome and quality of life in 152 patients. Hernia J Hernias Abdom Wall Surg. 2010; 14(6): 555‑60.